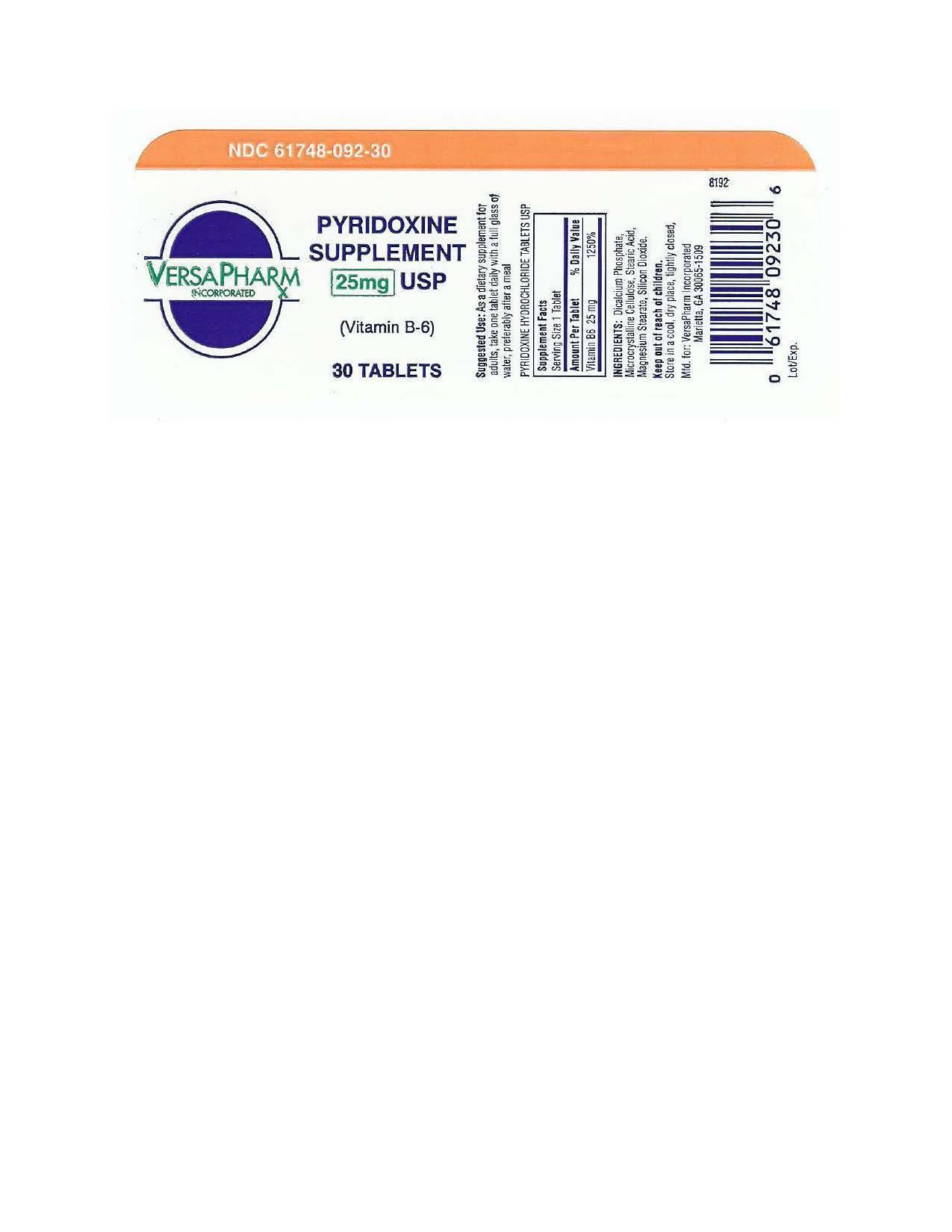
PYRIDOXINE HYDROCHLORIDE- pyridoxine hydrochloride tablet
VersaPharm Incorporated
----------
Pyridoxine Hydrochloride Tablets USP, 25 mg (Vitamin B6)
Supplement Facts
| Serving Size: 1 Tablet |
| Amount Per Tablet % Daily Value |
| Vitamin B6 25 mg (as Pyridoxine Hydrochloride) 1250 % |
| Other ingredients: Dicalcium Phosphate, Microcrystalline Cellulose, Stearic Acid, Magnesium Stearate and Silicon Dioxide. |
| PYRIDOXINE HYDROCHLORIDE
pyridoxine hydrochloride tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| shape | ||
| size (solid drugs) | 9 mm | |
| imprint | ||
| scoring | 1 | |
| Labeler - VersaPharm Incorporated (117696953) |
Revised: 3/2022
Document Id: 5ac3a70c-6ab9-4332-87e5-987be09d11d3
Set id: cca3c335-f43c-4053-b757-5ec48cf103c5
Version: 2
Effective Time: 20220311
VersaPharm Incorporated