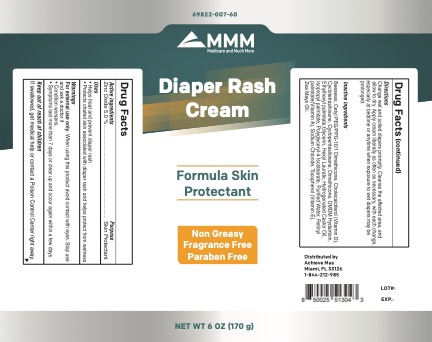
• Helps treat and prevent diaper rash.
• Protects chafed skin associated with diaper rash and helps protect from wetness.
For external use only. When using this product avoid contact with eyes. Stop use and ask a doctor if
- Condition worsens.
- Symptoms last more than 7 days or clear up and occur again within a few days.
If swallowed, get medical help or contact a Poison Control Center right away.
Change wet and soiled diapers promptly. Cleanse the affected area, and allow to dry. Apply cream liberally as often as necessary, with each change, especially at bedtime or anytime when exposure to wet diapers may be prolonged.
Stop use and ask a doctor if
• Condition worsens.
• Symptoms last more than 7 days or clear up and occur again within a few days.
Change wet and soiled diapers promptly. Cleanse the affected area, and allow to dry. Apply cream liberally as often as necessary, with each change, especially at bedtime or anytime when exposure to wet diapers may be prolonged.
Purified Water, cyclotetrasiloxane , Isopropyl palmitate, Ethylhexyl palmitate, Glycerin, Cyclopentasiloxane, Polyglyceryl-4 Isostearate, Cetyl PEG/PPG-10/1 Dimethicone, Hexyl Laurate, Dimethicone, Tocopherol (Vitamin E), Zea Mays Oil, Retinyl palmitate(Vitamin A), Cholecalciferol (Vitamin D), Beeswax, Hydrogenated Castor Oil, Sodium Chloride, DMDM Hydantoin.