DESCRIPTION
Levocarnitine is a carrier molecule in the transport of long-chain fatty acids across the inner mitochondrial membrane.
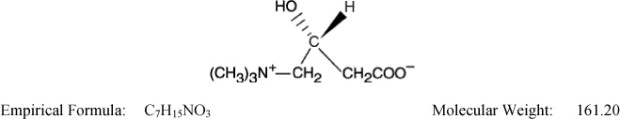
The chemical name of levocarnitine is 3-carboxy-2(R)-hydroxy-N,N,N-trimethyl-1-propanaminium, inner salt. Levocarnitine is a white crystalline, hygroscopic powder. It is readily soluble in water, hot alcohol, and insoluble in acetone. The specific rotation of levocarnitine is between –29° and –32°. Its chemical structure is:
Levocarnitine injection, USP is a sterile aqueous solution containing 1 g of levocarnitine per 5 mL vial, and 4 g of levocarnitine per 20 mL vial, and water for injection q.s. The pH is adjusted to 6 to 6.5 with hydrochloric acid or sodium hydroxide.
CLINICAL PHARMACOLOGY
Levocarnitine is a naturally occurring substance required in mammalian energy metabolism. It has been shown to facilitate long-chain fatty acid entry into cellular mitochondria, thereby delivering substrate for oxidation and subsequent energy production. Fatty acids are utilized as an energy substrate in all tissues except the brain. In skeletal and cardiac muscle, fatty acids are the main substrate for energy production.
Primary systemic carnitine deficiency is characterized by low concentrations of levocarnitine in plasma, RBC, and/or tissues. It has not been possible to determine which symptoms are due to carnitine deficiency and which are due to an underlying organic acidemia, as symptoms of both abnormalities may be expected to improve with levocarnitine. The literature reports that carnitine can promote the excretion of excess organic or fatty acids in patients with defects in fatty acid metabolism and/or specific organic acidopathies that bioaccumulate acylCoA esters.1-6
Secondary carnitine deficiency can be a consequence of inborn errors of metabolism or iatrogenic factors such as hemodialysis. Levocarnitine may alleviate the metabolic abnormalities of patients with inborn errors that result in accumulation of toxic organic acids. Conditions for which this effect has been demonstrated are: glutaric aciduria II, methyl malonic aciduria, propionic acidemia, and medium chain fatty acylCoA dehydrogenase deficiency.7,8 Autointoxication occurs in these patients due to the accumulations of acylCoA compounds that disrupt intermediary metabolism. The subsequent hydrolysis of the acylCoA compound to its free acid results in acidosis which can be life-threatening. Levocarnitine clears the acylCoA compound by formation of acylcarnitine, which is quickly excreted. Carnitine deficiency is defined biochemically as abnormally low plasma concentrations of free carnitine, less than 20 μmol/L at one week post term and may be associated with low tissue and/or urine concentrations. Further, this condition may be associated with a plasma concentration ratio of acylcarnitine/levocarnitine greater than 0.4 or abnormally elevated concentrations of acylcarnitine in the urine. In premature infants and newborns, secondary deficiency is defined as plasma levocarnitine concentrations below age-related normal concentrations.
End Stage Renal Disease (ESRD) patients on maintenance hemodialysis may have low plasma carnitine concentrations and an increased ratio of acylcarnitine/carnitine because of reduced intake of meat and dairy products, reduced renal synthesis and dialytic losses. Certain clinical conditions common in hemodialysis patients such as malaise, muscle weakness, cardiomyopathy and cardiac arrhythmias may be related to abnormal carnitine metabolism.
Pharmacokinetic and clinical studies with levocarnitine have shown that administration of levocarnitine to ESRD patients on hemodialysis results in increased plasma levocarnitine concentrations.
PHARMACOKINETICS
In a relative bioavailability study in 15 healthy adult male volunteers levocarnitine tablets were found to be bio-equivalent to levocarnitine oral solution. Following 4 days of dosing with 6 tablets of levocarnitine 330 mg twice daily or 2 g of levocarnitine oral solution twice daily, the maximum plasma concentration (Cmax) was about 80 μmol/L and the time to maximum plasma concentration (Tmax) occurred at 3.3 hours.
The plasma concentration profiles of levocarnitine after a slow 3 minute intravenous bolus dose of 20 mg/kg of levocarnitine were described by a two-compartment model. Following a single intravenous administration, approximately 76% of the levocarnitine dose was excreted in the urine during the 0 to 24 hour interval. Using plasma concentrations uncorrected for endogenous levocarnitine, the mean distribution half life was 0.585 hours and the mean apparent terminal elimination half life was 17.4 hours.
The absolute bioavailability of levocarnitine from the two oral formulations of levocarnitine, calculated after correction for circulating endogenous plasma concentrations of levocarnitine, was 15.1% ± 5.3% for levocarnitine tablets and 15.9% ± 4.9% for levocarnitine oral solution.
Total body clearance of levocarnitine (Dose/AUC including endogenous baseline concentrations) was a mean of 4.00 L/h.
Levocarnitine was not bound to plasma protein or albumin when tested at any concentration or with any species including the human.9
In a 9-week study, 12 ESRD patients undergoing hemodialysis for at least 6 months received levocarnitine 20 mg/kg three times per week after dialysis. Prior to initiation of levocarnitine therapy, mean plasma levocarnitine concentrations were approximately 20 μmol/L pre-dialysis and 6 μmol/L post-dialysis. The table summarizes the pharmacokinetic data (mean ± SD μmol/L) after the first dose of levocarnitine and after 8 weeks of levocarnitine therapy.
|
N=12 |
Baseline |
Single dose |
8 weeks |
|
Cmax |
- |
1139 ± 240 |
1190 ± 270 |
|
Trough (pre-dialysis, pre-dose) |
21.3 ± 7.7 |
68.4 ± 26.1 |
190 ± 55 |
After one week of levocarnitine therapy (3 doses), all patients had trough concentrations between 54 and 180 μmol/L (normal 40-50 μmol/L) and concentrations remained relatively stable or increased over the course of the study.
In a similar study in ESRD patients also receiving 20 mg/kg levocarnitine 3 times per week after hemodialysis, 12- and 24-week mean pre-dialysis (trough) levocarnitine concentrations were 189 (N=25) and 243 (N=23) μmol/L, respectively.
In a dose-ranging study in ESRD patients undergoing hemodialysis, patients received 10, 20, or 40 mg/kg levocarnitine 3 times per week following dialysis (N~30 for each dose group). Mean ± SD trough levocarnitine concentrations (μmol/L) by dose after 12 and 24 weeks of therapy are summarized in the table.
|
12 weeks |
24 weeks |
|
|
10 mg/kg |
116 ± 69 |
148 ± 50 |
|
20 mg/kg |
210 ± 58 |
240 ± 60 |
|
40 mg/kg |
371 ± 111 |
456 ± 162 |
While the efficacy of levocarnitine to increase carnitine concentrations in patients with ESRD undergoing dialysis has been demonstrated, the effects of supplemental carnitine on the signs and symptoms of carnitine deficiency and on clinical outcomes in this population have not been determined.
METABOLISM AND EXCRETION
In a pharmacokinetic study where five normal adult male volunteers received an oral dose of [3H-methyl]-L-carnitine following 15 days of a high carnitine diet and additional carnitine supplement, 58% to 65% of the administered radioactive dose was recovered in the urine and feces in 5 to 11 days. Maximum concentration of [3H-methyl]-L-carnitine in serum occurred from 2.0 to 4.5 hr after drug administration. Major metabolites found were trimethylamine N-oxide, primarily in urine (8% to 49% of the administered dose) and [3H]-γ-butyrobetaine, primarily in feces (0.44% to 45% of the administered dose). Urinary excretion of levocarnitine was about 4% to 8% of the dose. Fecal excretion of total carnitine was less than 1% of the administered dose.10
After attainment of steady state following 4 days of oral administration of levocarnitine tablets (1980 mg every 12 hours) or oral solution (2000 mg every 12 hours) to 15 healthy male volunteers, the mean urinary excretion of levocarnitine during a single dosing interval (12 hours) was about 9% of the orally administered dose (uncorrected for endogenous urinary excretion).
INDICATIONS AND USAGE
For the acute and chronic treatment of patients with an inborn error of metabolism which results in secondary carnitine deficiency.
For the prevention and treatment of carnitine deficiency in patients with end stage renal disease who are undergoing dialysis.
WARNINGS
Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, laryngeal edema, and bronchospasm have been reported following levocarnitine administration, mostly in patients with end stage renal disease who are undergoing dialysis. Some reactions occurred within minutes after intravenous administration of levocarnitine.
If a severe hypersensitivity reaction occurs, discontinue levocarnitine treatment and initiate appropriate medical treatment. Consider the risks and benefits of re-administering levocarnitine to individual patients following a severe reaction. If the decision is made to re-administer the product, monitor patients for a reoccurrence of signs and symptoms of a severe hypersensitivity reaction.
PRECAUTIONS
General
The safety and efficacy of oral levocarnitine has not been evaluated in patients with renal insufficiency. Chronic administration of high doses of oral levocarnitine in patients with severely compromised renal function or in ESRD patients on dialysis may result in accumulation of the potentially toxic metabolites, trimethylamine (TMA) and trimethylamine-N-oxide (TMAO), since these metabolites are normally excreted in the urine.
Drug Interactions
Reports of INR increase with the use of warfarin have been observed. It is recommended that INR levels be monitored in patients on warfarin therapy after the initiation of treatment with levocarnitine or after dose adjustments.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenicity tests performed in Salmonella typhimurium, Saccharomyces cerevisiae, and Schizosaccharomyces pombe indicate that levocarnitine is not mutagenic. No long-term animal studies have been performed to evaluate the carcinogenic potential of levocarnitine.
Pregnancy
Reproductive studies have been performed in rats and rabbits at doses up to 3.8 times the human dose on the basis of surface area and have revealed no evidence of impaired fertility or harm to the fetus due to levocarnitine. There are, however, no adequate and well controlled studies in pregnant women.
Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Levocarnitine supplementation in nursing mothers has not been specifically studied.
Studies in dairy cows indicate that the concentration of levocarnitine in milk is increased following exogenous administration of levocarnitine. In nursing mothers receiving levocarnitine, any risks to the child of excess carnitine intake need to be weighed against the benefits of levocarnitine supplementation to the mother. Consideration may be given to discontinuation of nursing or of levocarnitine treatment.
ADVERSE REACTIONS
Clinical Trials Experience
Transient nausea and vomiting have been observed. Less frequent adverse reactions are body odor, nausea, and gastritis. An incidence for these reactions is difficult to estimate due to the confounding effects of the underlying pathology.
The table below lists the adverse events that have been reported in two double-blind, placebo-controlled trials in patients on chronic hemodialysis. Events occurring at ≥ 5% are reported without regard to causality.
Adverse Events with a Frequency ≥ 5% Regardless of Causality by Body System
|
Placebo (n=63) |
Levocarnitine 10 mg (n=34) |
Levocarnitine 20 mg (n=62) |
Levocarnitine 40 mg (n=34) |
Levocarnitine 10, 20 & 40 mg (n=130) |
|
|
Body as Whole | |||||
|
Abdominal pain |
17 |
21 |
5 |
6 |
9 |
|
Accidental injury |
10 |
12 |
8 |
12 |
10 |
|
Allergic reaction |
5 |
6 |
2 |
||
|
Asthenia |
8 |
9 |
8 |
12 |
9 |
|
Back pain |
10 |
9 |
8 |
6 |
8 |
|
Chest pain |
14 |
6 |
15 |
12 |
12 |
|
Fever |
5 |
6 |
5 |
12 |
7 |
|
Flu syndrome |
40 |
15 |
27 |
29 |
25 |
|
Headache |
16 |
12 |
37 |
3 |
22 |
|
Infection |
17 |
15 |
10 |
24 |
15 |
|
Injection site reaction |
59 |
38 |
27 |
38 |
33 |
|
Pain |
49 |
21 |
32 |
35 |
30 |
|
Cardiovascular | |||||
|
Arrhythmia |
5 |
3 |
3 |
2 |
|
|
Atrial fibrillation |
2 |
6 |
2 |
||
|
Cardiovascular disorder |
6 |
3 |
5 |
6 |
5 |
|
Electrocardiogram abnormal |
3 |
6 |
2 |
||
|
Hemorrhage |
6 |
9 |
2 |
3 |
4 |
|
Hypertension |
14 |
18 |
21 |
21 |
20 |
|
Hypotension |
19 |
15 |
19 |
3 |
14 |
|
Palpitations |
3 |
8 |
5 |
||
|
Tachycardia |
5 |
6 |
5 |
9 |
6 |
|
Vascular disorder |
2 |
2 |
6 |
2 |
|
|
Digestive | |||||
|
Anorexia |
3 |
3 |
5 |
6 |
5 |
|
Constipation |
6 |
3 |
3 |
3 |
3 |
|
Diarrhea |
19 |
9 |
10 |
35 |
16 |
|
Dyspepsia |
10 |
9 |
6 |
5 |
|
|
Gastrointestinal disorder |
2 |
3 |
6 |
2 |
|
|
Melena |
3 |
6 |
2 |
||
|
Nausea |
10 |
9 |
5 |
12 |
8 |
|
Stomach atony |
5 | ||||
|
Vomiting |
16 |
9 |
16 |
21 |
15 |
|
Endocrine System | |||||
|
Parathyroid disorder |
2 |
6 |
2 |
6 |
4 |
|
Hemic/Lymphatic | |||||
|
Anemia |
3 |
3 |
5 |
12 |
6 |
|
Metabolic/Nutritional | |||||
|
Hypercalcemia |
3 |
15 |
8 |
6 |
9 |
|
Hyperkalemia |
6 |
6 |
6 |
6 |
6 |
|
Hypervolemia |
17 |
3 |
3 |
12 |
5 |
|
Peripheral edema |
3 |
6 |
5 |
3 |
5 |
|
Weight decrease |
3 |
3 |
8 |
3 |
5 |
|
Weight increase |
2 |
3 |
6 |
2 |
|
|
Musculo-Skeletal | |||||
|
Leg cramps |
13 |
8 |
4 |
||
|
Myalgia |
6 | ||||
|
Nervous | |||||
|
Anxiety |
5 |
2 |
1 |
||
|
Depression |
3 |
6 |
5 |
6 |
5 |
|
Dizziness |
11 |
18 |
10 |
15 |
13 |
|
Drug dependence |
2 |
6 |
2 |
||
|
Hypertonia |
5 |
3 |
1 |
||
|
Insomnia |
6 |
3 |
6 |
4 |
|
|
Paresthesia |
3 |
3 |
3 |
12 |
5 |
|
Vertigo |
6 |
2 |
|||
|
Respiratory | |||||
|
Bronchitis |
5 |
3 |
3 |
||
|
Cough increase |
16 |
10 |
18 |
9 |
|
|
Dyspnea |
19 |
3 |
11 |
3 |
7 |
|
Pharyngitis |
33 |
24 |
27 |
15 |
23 |
|
Respiratory disorder |
5 | ||||
|
Rhinitis |
10 |
6 |
11 |
6 |
9 |
|
Sinusitis |
5 |
2 |
3 |
2 |
|
|
Skin And Appendages | |||||
|
Pruritus |
13 |
8 |
3 |
5 |
|
|
Rash |
3 |
5 |
3 |
3 |
|
|
Special Senses | |||||
|
Amblyopia |
2 |
6 |
3 |
||
|
Eye disorder |
3 |
6 |
3 |
3 |
|
|
Taste perversion |
2 |
9 |
3 |
||
|
Urogenital | |||||
|
Urinary tract infect |
6 |
3 |
3 |
2 |
|
|
Kidney failure |
5 |
6 |
6 |
6 |
6 |
Postmarketing Experience
The following adverse reactions have been reported:
Neurologic Reactions: Seizures have been reported to occur in patients, with or without pre-existing seizure activity, receiving either oral or intravenous levocarnitine. In patients with pre-existing seizure activity, an increase in seizure frequency and/or severity has been reported.
Hypersensitivity reactions: Anaphylaxis, laryngeal edema and bronchospasm (see WARNINGS).
OVERDOSAGE
There have been no reports of toxicity from levocarnitine overdosage. Levocarnitine is easily removed from plasma by dialysis. The intravenous LD50 of levocarnitine in rats is 5.4 g/kg and the oral LD50 of levocarnitine in mice is 19.2 g/kg. Large doses of levocarnitine may cause diarrhea.
DOSAGE AND ADMINISTRATION
Levocarnitine Injection, USP is administered intravenously.
Metabolic Disorders
The recommended dose is 50 mg/kg given as a slow 2 to 3 minute bolus injection or by infusion. Often a loading dose is given in patients with severe metabolic crisis, followed by an equivalent dose over the following 24 hours. It should be administered every 3 hours or every 4 hours, and never less than every 6 hours either by infusion or by intravenous injection. All subsequent daily doses are recommended to be in the range of 50 mg/kg or as therapy may require. The highest dose administered has been 300 mg/kg.
It is recommended that a plasma carnitine concentration be obtained prior to beginning this parenteral therapy. Weekly and monthly monitoring is recommended as well. This monitoring should include blood chemistries, vital signs, plasma carnitine concentrations (the plasma free carnitine concentration should be between 35 and 60 μmol/L) and overall clinical condition.
ESRD Patients on Hemodialysis
The recommended starting dose is 10 to 20 mg/kg dry body weight as a slow 2 to 3 minute bolus injection into the venous return line after each dialysis session. Initiation of therapy may be prompted by trough (pre-dialysis) plasma levocarnitine concentrations that are below normal (40 to 50 µmol/L). Dose adjustments should be guided by trough (pre-dialysis) levocarnitine concentrations, and downward dose adjustments (e.g. to 5 mg/kg after dialysis) may be made as early as the third or fourth week of therapy.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
COMPATIBILITY AND STABILITY
Levocarnitine Injection, USP is compatible and stable when mixed in parenteral solutions of Sodium Chloride 0.9% or Lactated Ringer's in concentrations ranging from 250 mg/500 mL (0.5 mg/mL) to 4200 mg/500 mL (8 mg/mL) and stored at room temperature (25°C) for up to 24 hours in PVC plastic bags.
HOW SUPPLIED:
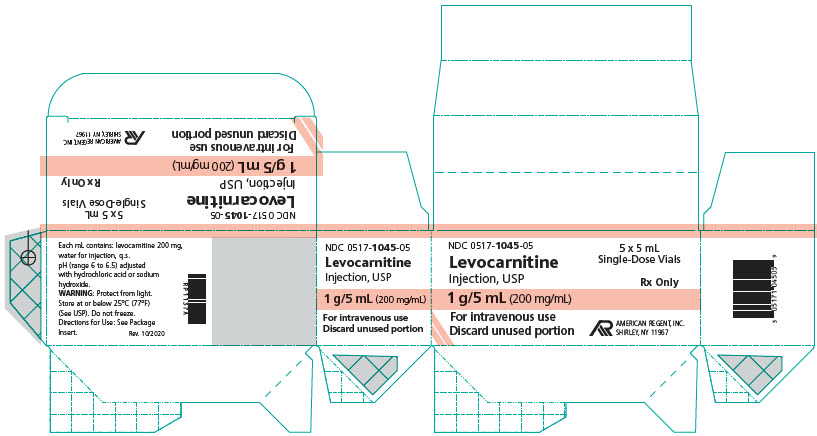
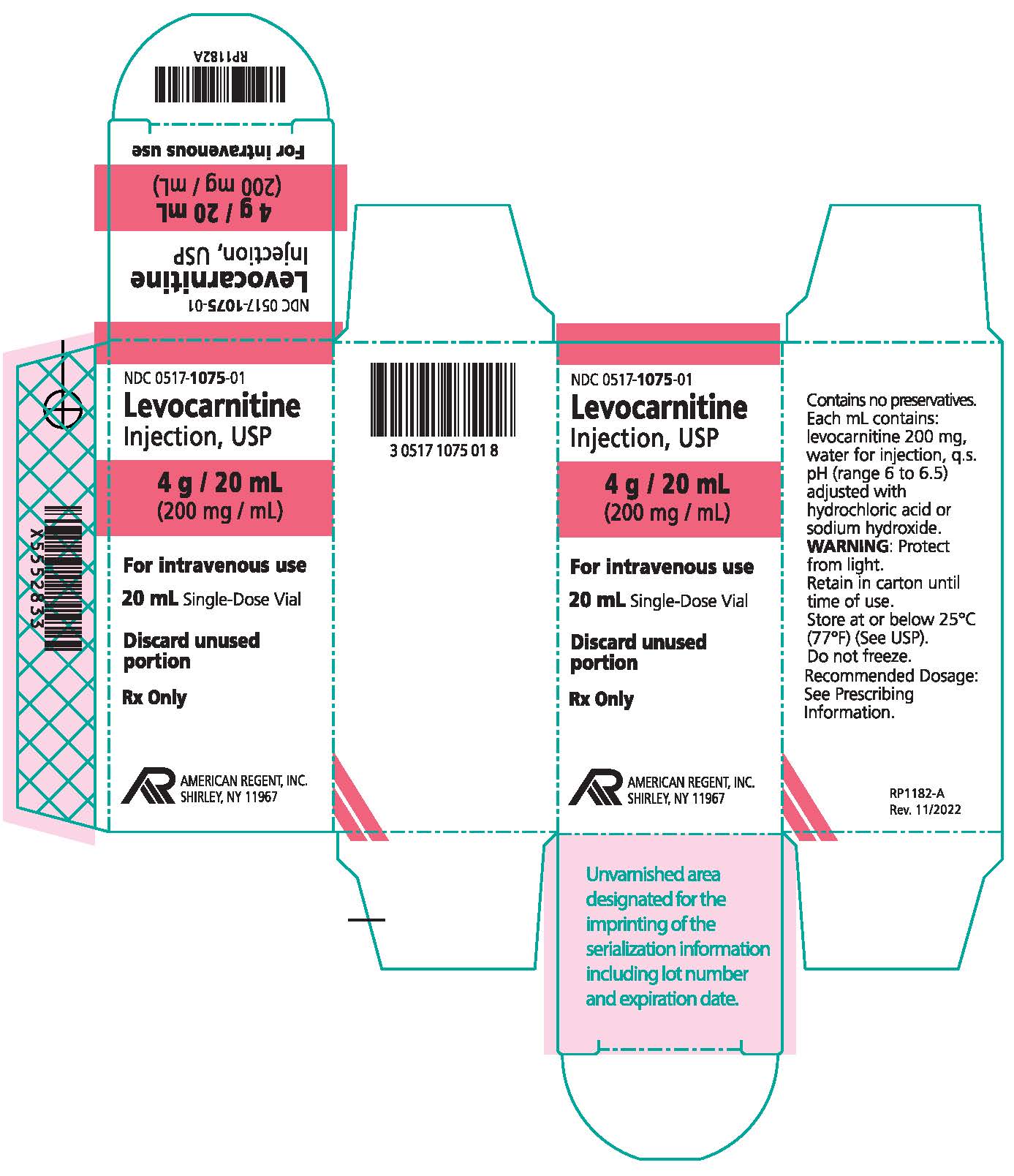
How supplied: Levocarnitine Injection, USP is available in 5 mL and 20 mL single-dose vials. Each 5 mL vial contains 1 g/5 mL and each 20 mL vial contains 4 g/20 mL (200 mg/mL).
NDC 0517-1045-05 1 gram/5 mL (200 mg/mL) Single-Dose Vial Packages of 5
NDC 0517-1075-01 4 grams/20 mL (200 mg/mL) Single-Dose Vial Individually boxed
Store at or below 25°C (77°F) (See USP). Do not freeze. Store vials in carton until their use to protect from light. Discard unused portion of an opened vial, as the formulation does not contain a preservative.
REFERENCES
- Bohmer, T., Rydning, A. and Solberg, H.E. 1974. Carnitine levels in human serum in health and disease. Clin. Chim. Acta 57:55-61.
- Brooks, H., Goldberg L., Holland, R. et al. 1977. Carnitine-induced effects on cardiac and peripheral hemodynamics. J. Clin. Pharmacol. 17:561-568.
- Christiansen, R., Bremer, J. 1976. Active transport of butyrobetaine and carnitine into isolated liver cells. Biochim. Biophys. Acta 448:562-577.
- Lindstedt, S. and Lindstedt, G. 1961. Distribution and excretion of carnitine in the rat. Acta Chem. Scand. 15:701-702.
- Rebouche, C.J. and Engel, A.G. 1983. Carnitine metabolism and deficiency syndromes. Mayo Clin. Proc. 58:533-540.
- Rebouche, C.J. and Paulson, D.J. 1986. Carnitine metabolism and function in humans. Ann. Rev. Nutr. 6:41-66.
- Scriver, C.R., Beaudet, A.L., Sly, W.S. and Valle, D. 1989. The Metabolic Basis of Inherited Disease. New York: McGraw-Hill.
- Schaub, J., Van Hoof, F. and Vis, H.L. 1991. Inborn Errors of Metabolism. New York: Raven Press.
- Marzo, A., Arrigoni Martelli, E., Mancinelli, A., Cardace, G., Corbelletta, C., Bassani, E., and Solbiati, M. 1991. Protein binding of L-carnitine family components. Eur. J. Drug Met. Pharmacokin., Special Issue III: 364-368.
- Rebouche, C.J. 1991. Quantitative estimation of absorption and degradation of a carnitine supplement by human adults. Metabolism 40:1305-1310.
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
RQ1094-C
Rev. 01/2022
PRINCIPAL DISPLAY PANEL - 5 mL Container
NDC 0517-1045-01
Rx Only
Levocarnitine Injection, USP
1 g/5 mL (200 mg/mL)
For intravenous use
Discard unused portion
5 mL Single-Dose Vial
AMERICAN REGENT, INC.
SHIRLEY, NY 11967

PRINCIPAL DISPLAY PANEL - 5 mL Carton
NDC 0517-1045-05
5 x 5 mL Single-Dose Vials
Rx Only
Levocarnitine Injection, USP
1 g/5 mL (200 mg/mL)
5 x 5 mL Single-Dose Vials
For intravenous use
Discard unused portion
AMERICAN REGENT, INC.
SHIRLEY, NY 11967