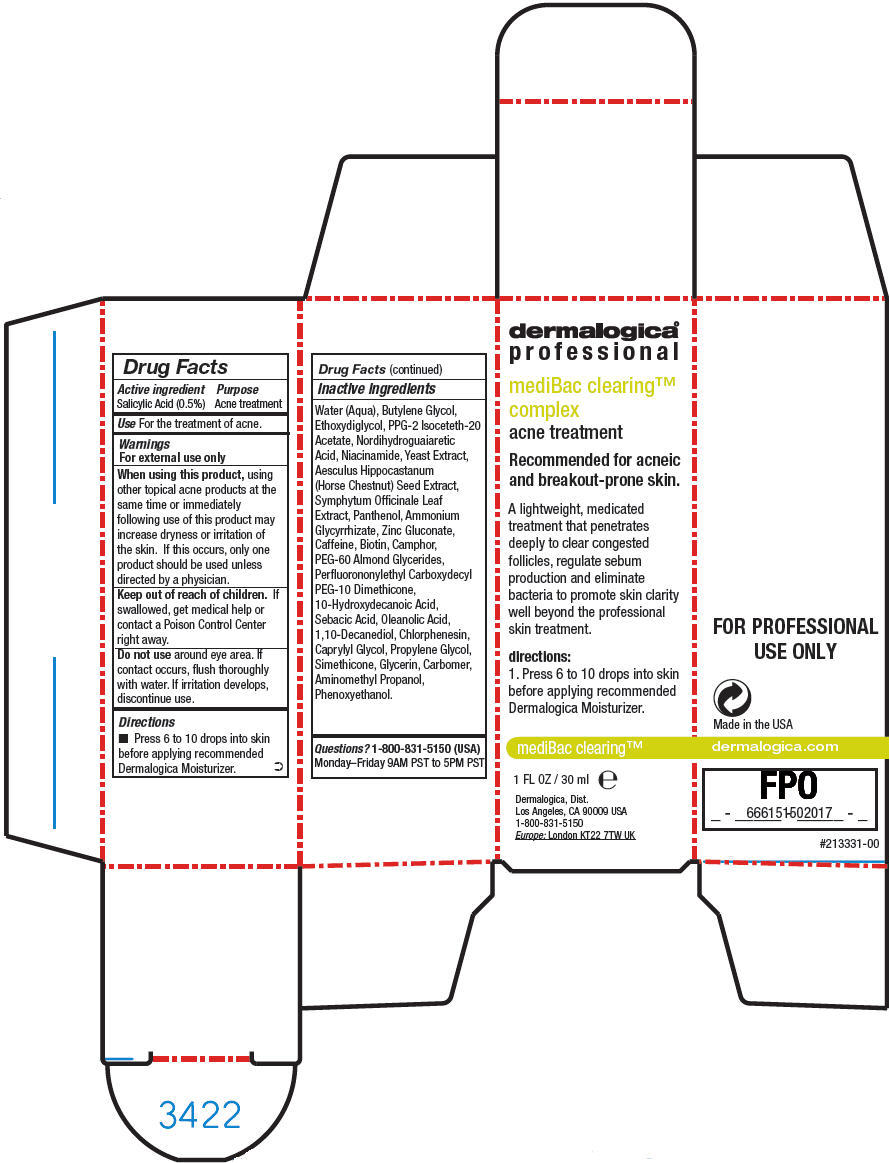
Warnings
For external use only
When using this product, using other topical acne products at the same time or immediately following use of this product may increase dryness or irritation of the skin. If this occurs, only one product should be used unless directed by a physician.
Inactive ingredients
Water (Aqua), Butylene Glycol, Ethoxydiglycol, PPG-2 Isoceteth-20 Acetate, Nordihydroguaiaretic Acid, Niacinamide, Yeast Extract, Aesculus Hippocastanum (Horse Chestnut) Seed Extract, Symphytum Officinale Leaf Extract, Panthenol, Ammonium Glycyrrhizate, Zinc Gluconate, Caffeine, Biotin, Camphor, PEG-60 Almond Glycerides, Perfluorononylethyl Carboxydecyl PEG-10 Dimethicone, 10-Hydroxydecanoic Acid, Sebacic Acid, Oleanolic Acid, 1,10-Decanediol, Chlorphenesin, Caprylyl Glycol, Propylene Glycol, Simethicone, Glycerin, Carbomer, Aminomethyl Propanol, Phenoxyethanol.
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
dermalogica®
professional
mediBac clearing™
complex
acne treatment
Recommended for acneic
and breakout-prone skin.
A lightweight, medicated
treatment that penetrates
deeply to clear congested
follicles, regulate sebum
production and eliminate
bacteria to promote skin clarity
well beyond the professional
skin treatment.
directions:
1. Press 6 to 10 drops into skin
before applying recommended
Dermalogica Moisturizer.
mediBac clearing™
1 FL OZ / 30 ml e
Dermalogica, Dist.
Los Angeles, CA 90009 USA
1-800-831-5150
Europe: London KT22 7TW UK