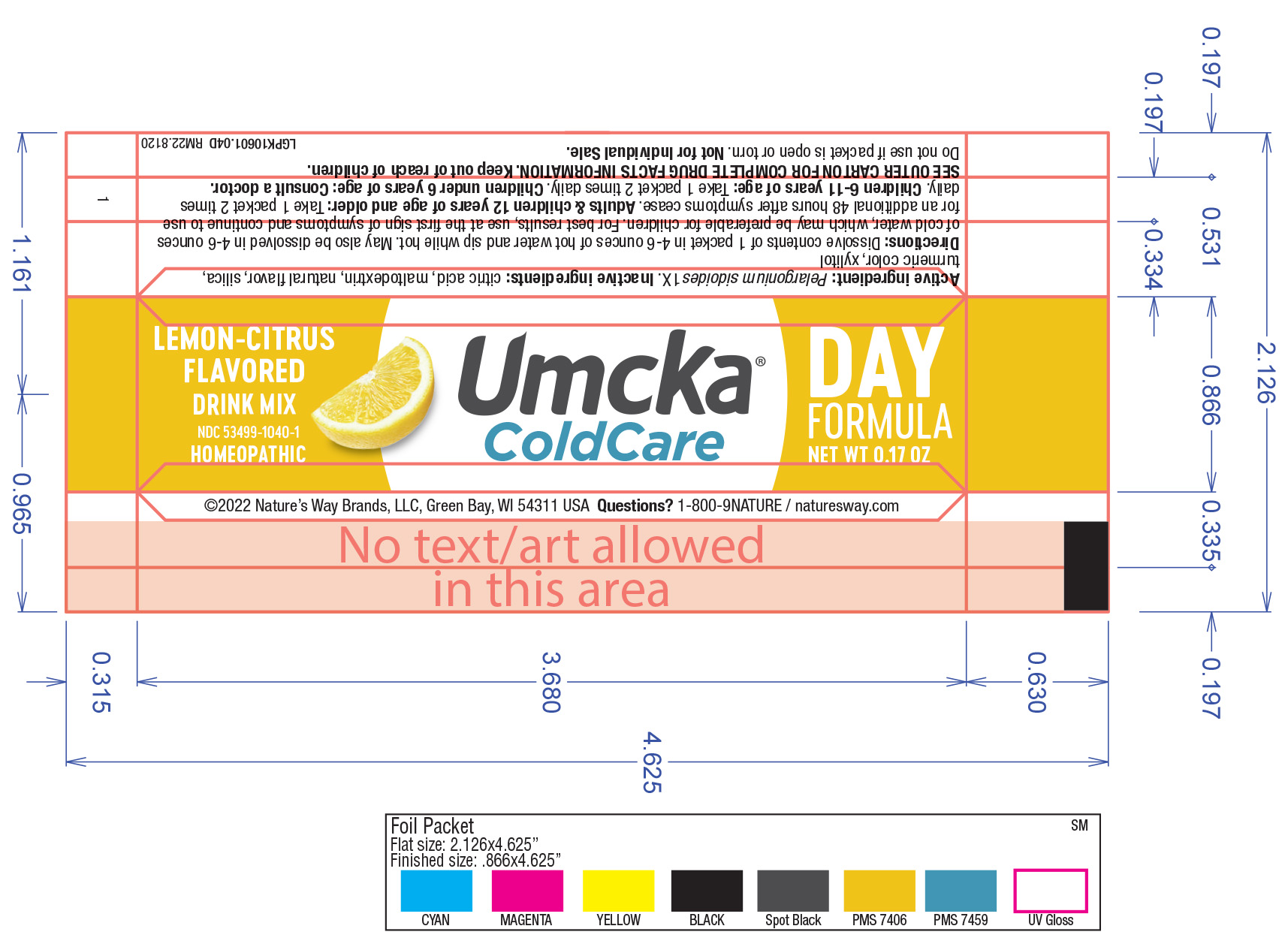
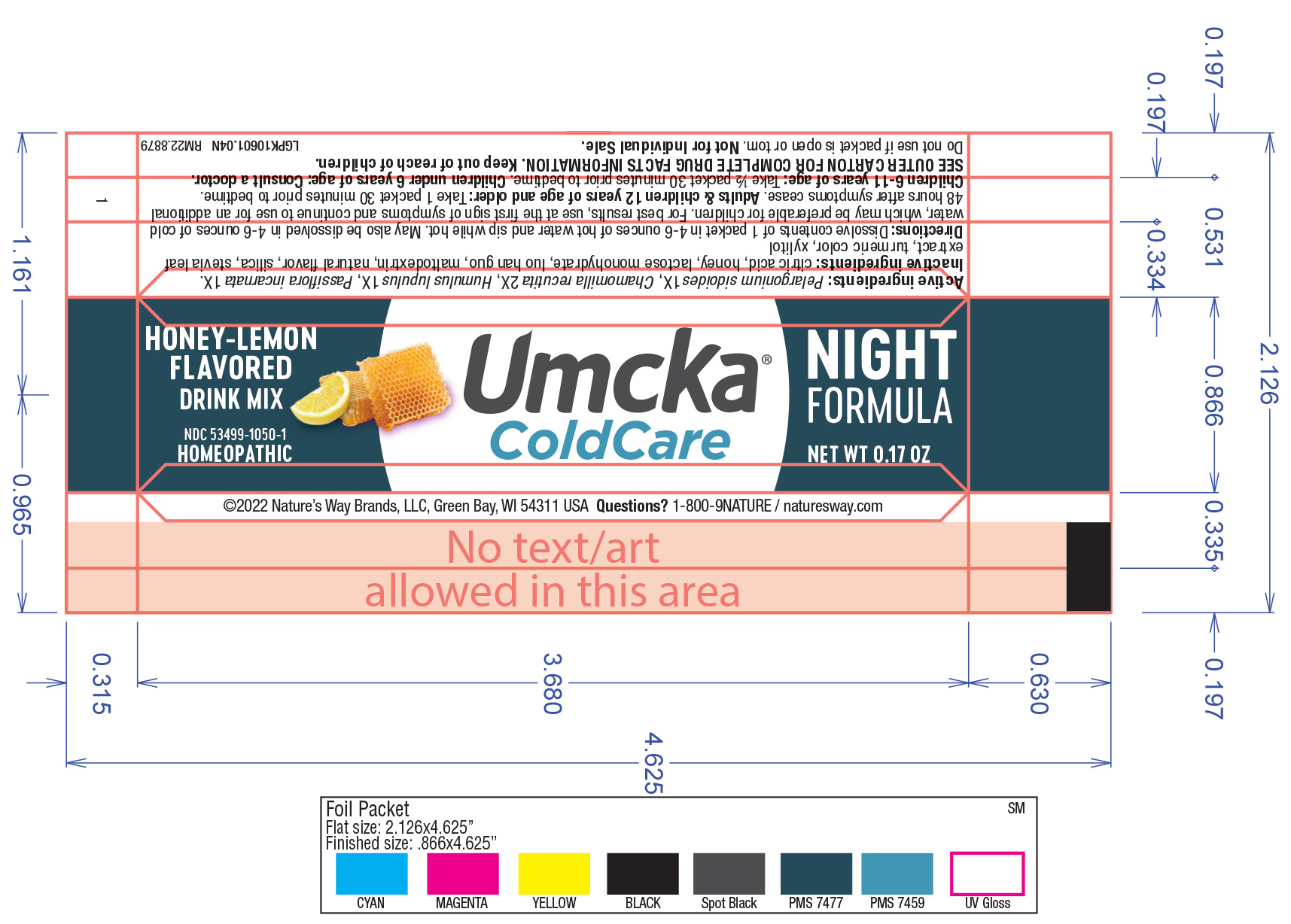
Active Ingredient
Umcka CC Day: Pelargonium sidoides 1X
Umcka CC Night: Pelargonium sidoides 1X
Chamomilla recutila 2X
Humulus Lupulus 1X
Passiflora incarnate 1X
Inactive Ingredient
Umcka CC Day: Citric acid, maltodextrin, natural flavor, silica, turmeric color, xylitol.
Umcka CC Night: Citric acid, honey, lactose monohydrate, lou han guo, maltodextrin, natural flavor, silica, stevia leaf extract, turmeric color, xylitol.
Doage & Administration
Directions
Umcka CC Day: Dissolve contents of 1 packet in 4-6 ounces of hot water and sip while hot. May also be dissolved in 4-6 ounces of cold water, which may be preferable for children. For best results, use at the first sign of symptoms and continue to use for an additional 48 hours after symptoms cease.
Adults/Children 12 years of age and older: Take 1 packet 2 times daily
Children 6-11 years of age: Take 1 packet 2 times daily
Children under 6 years of age: Consult a doctor
Umcka CC Night: Dissolve contents of 1 packet in 4-6 ounces of hot water and sip while hot. May also be dissolved in 4-6 ounces of cold water, which may be preferable for children. For best results, use at the first sign of symptoms and continue to use for an additional 48 hours after symptoms cease.
Adults/Children 12 years of age and older: Take 1 packet 30 minutes prior to bedtime
Children 6-11 years of age: Take 1/2 packet 30 minutes prior to bedtime
Children under 6 years of age: Consult a doctor
Indications & Usage
Umcka CC Day: Shortens duration and reduces severity of symptoms associated with common colds and throat/nasal/bronchial irritations: congestion, cough, hoarseness, sore throat
Umcka CC Night: Shortens duration and reduces severity of symptoms associated with common colds and throat/nasal/bronchial irritations: congestion, cough, headache, hoarseness, sore throat
Purpose
Umcka CC Day: Shortens duration and reduces severity of symptoms associated with common colds and throat/nasal/bronchial irritations: congestion, cough, hoarseness, sore throat
Umcka CC Night: Shortens duration and reduces severity of symptoms associated with common colds and throat/nasal/bronchial irritations: congestion, cough, headache, hoarseness, sore throat
Warnings
Umcka CC Day: Sore throat warning: severe or presistent sore throat for more than 2 days or if accompanied by high fever, headache, nausea, vomiting or rash may be serious. Consult a physician promptly.
Umcka CC Night: Sore throat warning: severe or presistent sore throat for more than 2 days or if accompanied by high fever, headache, nausea, vomiting or rash may be serious. Consult a physician promptly.
Ask Doctor
Umcka CC Day: Ask a doctor before use if you have a persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema, have a cough that is accompanied by excessive phlegm (mucus), are taking any medications, have any allergy to plants of the Geraniaceae family.
Umcka CC Night: Ask a doctor before use if you have a persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema, have a cough that is accompanied by excessive phlegm (mucus), are taking any medications, have any allergy to plants of the Geraniaceae family.
Stop Use
Umcka CC Day: Stop use and ask a doctor if new symptoms occur, symptoms worsen or do not get better within 7 days, fever worsens or lasts more than 3 days, cough lasts more than 7 days or occurs with rash or persistent headache.
These could be signs of a serious condition.
Umcka CC Night: Stop use and ask a doctor if new symptoms occur, symptoms worsen or do not get better within 7 days, fever worsens or lasts more than 3 days, cough lasts more than 7 days or occurs with rash or persistent headache.
These could be signs of a serious condition.
Pregnancy or Breast feeding
Umcka CC Day: If pregnant or breast-feeding, ask a healthcare professional before use.
Umcka CC Night: If pregnant or breast-feeding, ask a healthcare professional before use.
Keep out of reach of children.
Umcka CC Day: Keep out of reach of children.
Umcka CC Night: Keep out of reach of children.