FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Lymphatic Vessel Delineation
Lymphazuirn™ 1% (isosulfan blue) upon subcutaneous administration, delineates lymphatic vessels draining the region of injection. It is an adjunct to lymphography in: primary and secondary lymphedema of the extremities; chyluria, chylous ascites or chylothorax; lymph node involvement by primary or secondary neoplasm; and lymph node response to therapeutic modalities.
4 CONTRAINDICATIONS
Lymphazurin™ 1% (isosulfan blue) is contraindicated in those individuals with known hypersensitivity to triphenylmethane or related compounds.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Life-threatening anaphylactic reactions (respiratory distress, shock, angioedema) have occurred after Lymphazurin 1% administration. Reactions are more likely to occur in patients with a history of bronchial asthma, allergies, drug reactions or previous reactions to triphenylmethane dyes. Monitor patients closely for at least 60 minutes after administration of Lymphazurin 1%. Trained personnel should be available to administer emergency care including resuscitation.
5.2 Precipitation of Lymphazurin 1% by Lidocaine
The admixture of Lymphazurin 1% (with local anesthetics (i.e. lidocaine)) in the same syringe results in an immediate precipitation of 4 – 9% drug complex. Use a separate syringe to administer a local anesthetic.
5.3 Interference with Oxygen Saturation and Methemoglobin Measurements
Lymphazurin 1% interferes with measurements of oxygen saturation in peripheral blood by pulse oximetry and can cause falsely low readings. The interference effect is maximal at 30 minutes and minimal generally by four hours after administration. Arterial blood gas analysis may be needed to verify decreased arterial partial pressure of oxygen.
Lymphazurin 1% may also cause falsely elevated readings of methemoglobin by arterial blood gas analyzer. Therefore, co-oximetry may be needed to verify methemoglobin level.
6 ADVERSE REACTIONS
6.1 Postmarketing Experience
Hypersensitivity Reactions: Case series report an overall incidence of hypersensitivity reactions in approximately 2% of patients. Life-threatening anaphylactic reactions have occurred. Manifestations include respiratory distress, shock, angioedema, urticaria, pruritus. A death has been reported following administration of a similar compound employed to estimate the depth of a severe burn. Reactions are more likely to occur in patients with a personal or family history of bronchial asthma, significant allergies, drug reactions or previous reactions to triphenylmethane dyes [see Warnings and Precautions (5)].
Laboratory tests: Lymphazurin 1% interferes with measurements of oxygen saturation by pulse oximetry and of methemoglobin by gas analyzer [see Warnings and Precautions (5)].
Skin: transient or long-term (tattooing) blue coloration.
8 USE IN SPECIFIC POPULATIONS
10 OVERDOSAGE
Do not exceed indicated recommended dosage as overdosage levels have not been identified for Lymphazurin 1%.
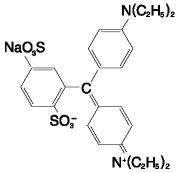
11 DESCRIPTION
The chemical name of Lymphazurin 1% (isosulfan blue) is N-[4-[[4-(diethylamino)phenyl] (2,5-disulfophenyl) methylene]-2,5-cyclohexadien-1-ylidene]-N-ethylehananamunium hydroxide, inner salt, sodium salt. Its structural formula is:
Lymphazurin 1% is a sterile aqueous solution for subcutaneous administration. Phosphate buffer in sterile, pyrogen free water is added in sufficient quantity to yield a final pH of 6.8-7.4. Each ml of solution contains 10 mg Isosulfan blue, 6.6 mg sodium monohydrogen phosphate and 2.7 mg potassium dihydrogen phosphate. The solution contains no preservative. Lymphazurin 1% is a contrast agent for the delineation of lymphatic vessels.
12 CLINICAL PHARMACOLOGY
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of Lymphazurin 1%. Reproduction studies in animals have not been conducted and, therefore, it is unknown if a problem concerning mutagenesis or impairment of fertility in either males or females exists.
13.2 Teratogenic Effects
Pregnancy Category C. Animal reproduction studies have not been conducted with Lymphazurin 1%. It is not known whether Lymphazurin 1% can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Lymphazurin 1% should be given to a pregnant woman only if clearly needed.
16 HOW SUPPLIED/STORAGE AND HANDLING
Lymphazurin 1% is supplied as a 5 ml single dose vial, 1% aqueous solution in a phosphate buffer prepared by appropriate manufacturing to be sterile and pyrogen-free.
17 PATIENT COUNSELING INFORMATION
Inform patients that urine color may be blue for 24 hours following administration of Lymphazurin 1%.
PRINCIPAL DISPLAY PANEL
D.I.N. 00592358
NDC 63261-250-21 LYM-100
LYMPHAZURIN* 1%
(isosulfan blue) Single Dose Vial 5 ml.
Mfd. for United States Surgical, a division of
Tyco Healthcare Group LP,
Norwalk, CT 06856 USA.
by Ben Venue Labs, Inc., Bedford, OH 44146 USA
Distributed in Canada by: Tyco Healthcare
Montreal, Quebec
Canada, H9R 5H8