Drug Facts
Active ingredients ( in each 5 mL teaspoonful)
Dextromethorphan Hydrobromide 10mg
Guaifenesin 100mg
Phenylephrine Hydrochloride 5mg
Uses
Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- cough due to minor throat and bronchial irritation
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes and make coughs more productive
- nasal congestion
- reduces swelling of nasal passages
Do not use this product
- If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emtional conditions, or Parkinson's disease). or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- a cough that occurs with too much phlegm (mucus)
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness, occur
- cough or nasal congestion persists for more than 1 week, trends to recur, or persistent headache. A persistent cough may be a sign of a serious condition.
- new symptoms occur
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Do not exceed recommeneded dosage.
- Use with enclosed dosage cup.
Adults and children 12 years of age and over------2 teaspoonfuls ( 2 TSP) every 4 hours, not to exceed 6 doses in 24 hours.
Children 6 to under 12 years of age........1 teaspoonful (1 TSP) every 4 hours, not to exceed 6 doses in 24 hours.
Children under 6 years of age....... Consult a doctor

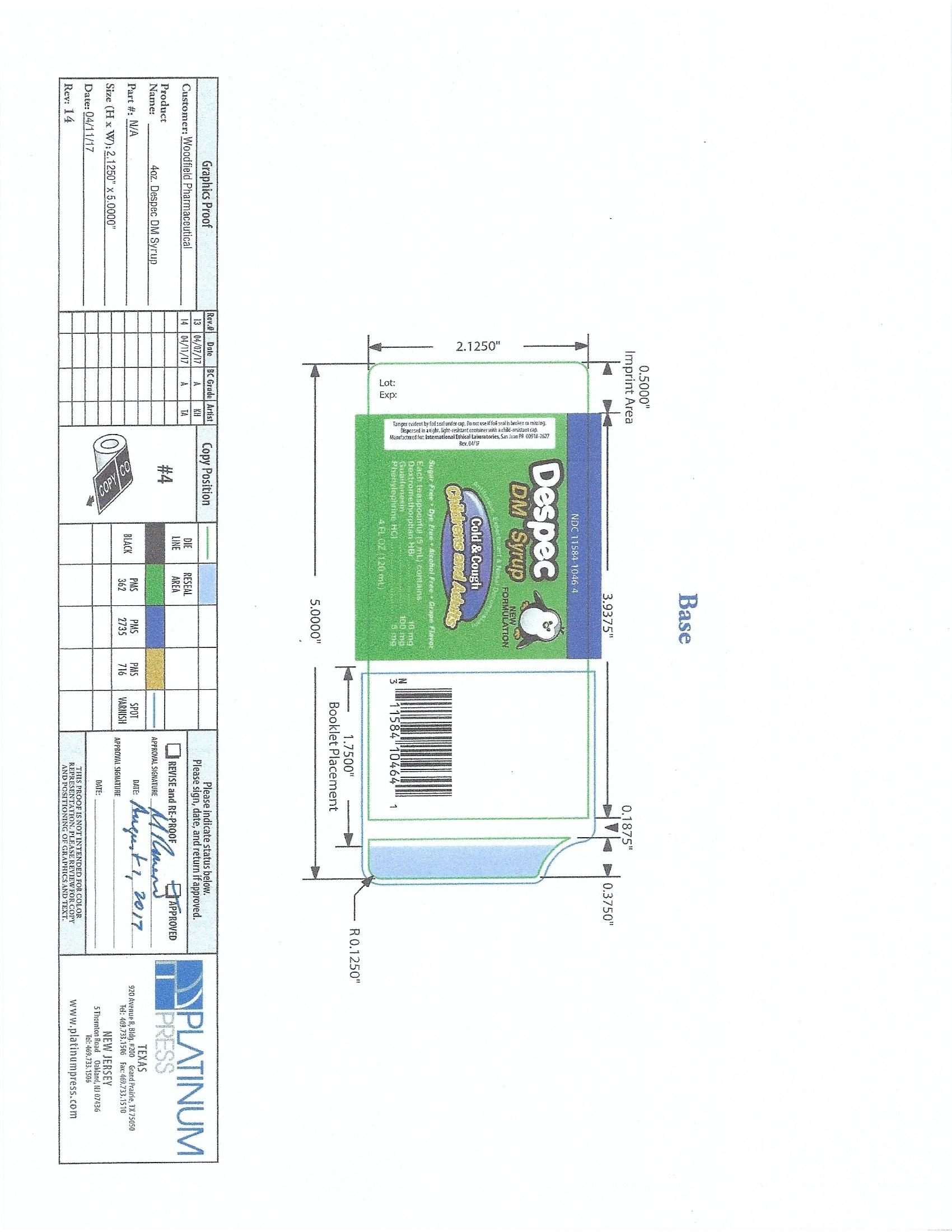 Principal Display Panel-120 mL Bottle Label
Principal Display Panel-120 mL Bottle Label