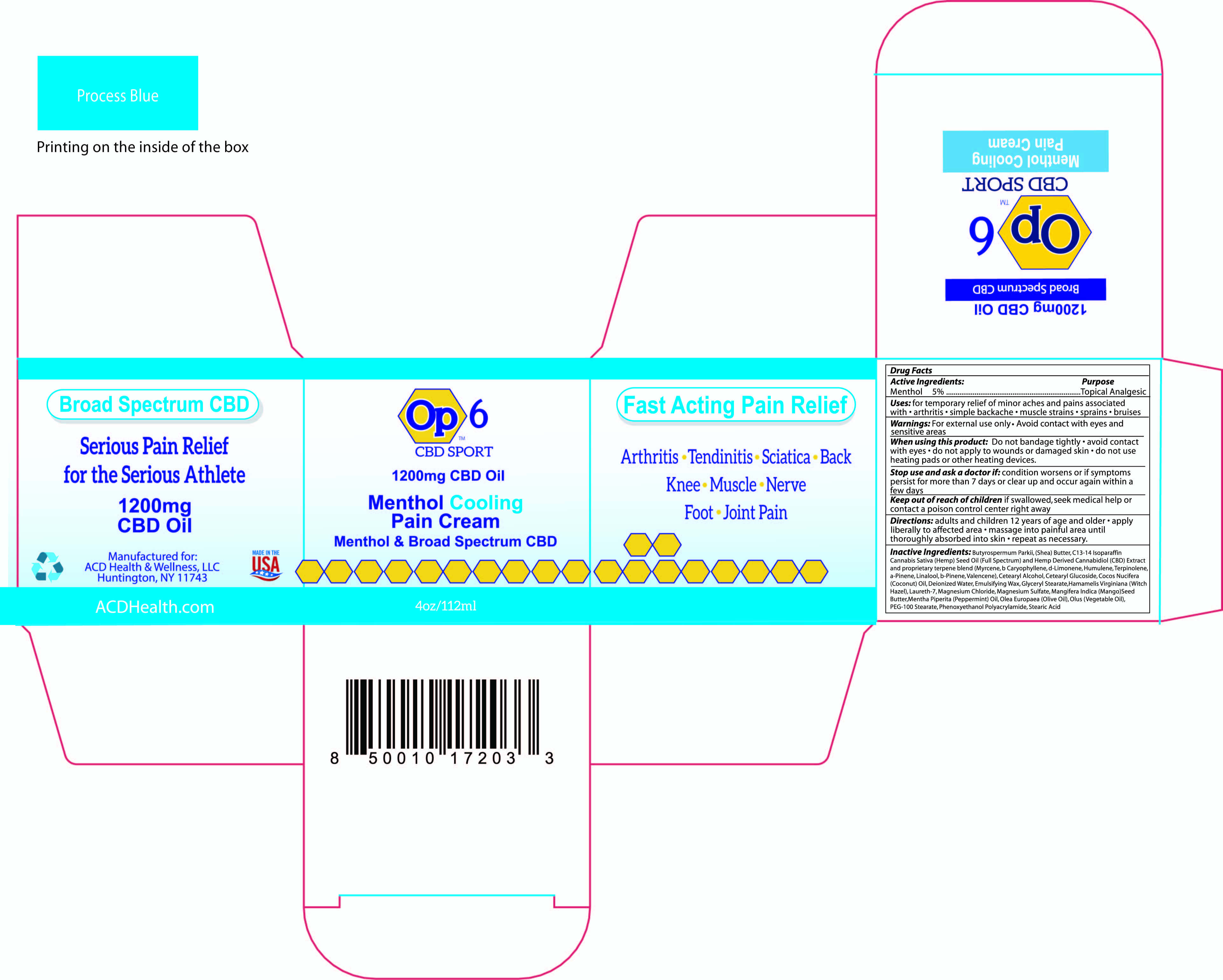
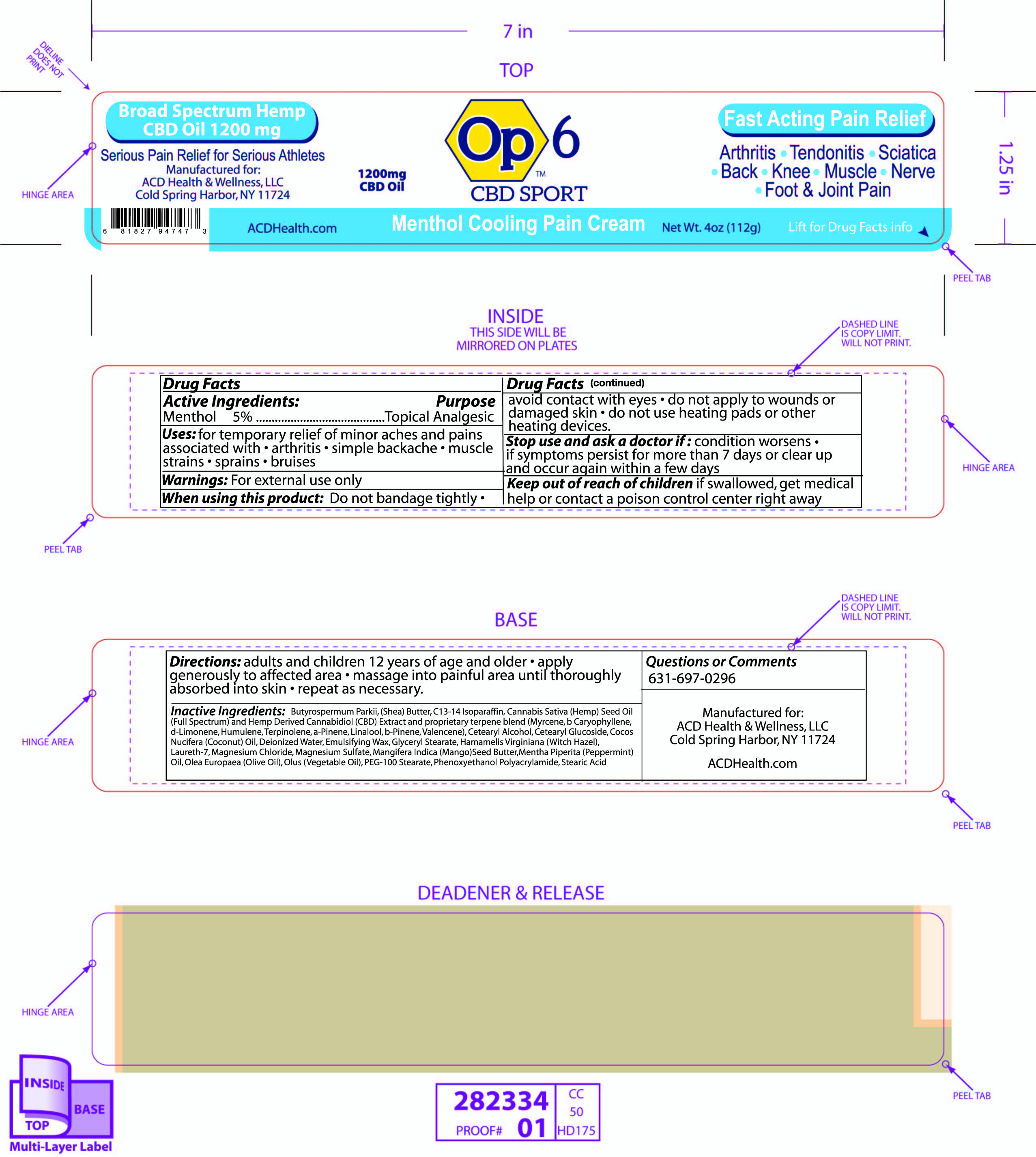
Warnings
For external use only
When using this product do not bandage tightly
avoid contact with eye
do not apply to wounds or damaged skin
Stop use and ask doctor
if condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children
If swallowed get medical help or contact Poison Control Center right away
Directions
adults and children 12 years of age and older
apply generously to affected area
massage into painful area until thoroughly absorbed into skin
repeat as necessary
Butyrospermum Parkii (Shea) Butter, C13-14 Isoparaffin, Cannabis Sativa (Hempseed) Oil and Hemp Derived Cannabidiol (CBD) Extract and proprietary terpene blend (Myrcene, b-Caryophyllene, d-Limonene, Humulene, Terpinolene, a-Pinene, Linalool, b-Pinene, Valencene, Cetearyl Alcohol, Cetearyl Glucoside, Cocos Nucifera (Coconut) Oil, Deionized Water, Emulsifying Wax, Glyceryl Stearate, Grapefruit Oil, Hamamelis Virginiana (Witch Hazel), Laureth-7, Magnesium Chloride, Magnesium Sulfate, Mangifera Indica (Mango) Seed Butter, Mentha Piperita (Peppermint) Oil, Olea Europaea (Olive) Oil, Olus (Vegetable) Oil, PEG-100, Phenoxyethanol, Polyacrylamide, Stearic Acid