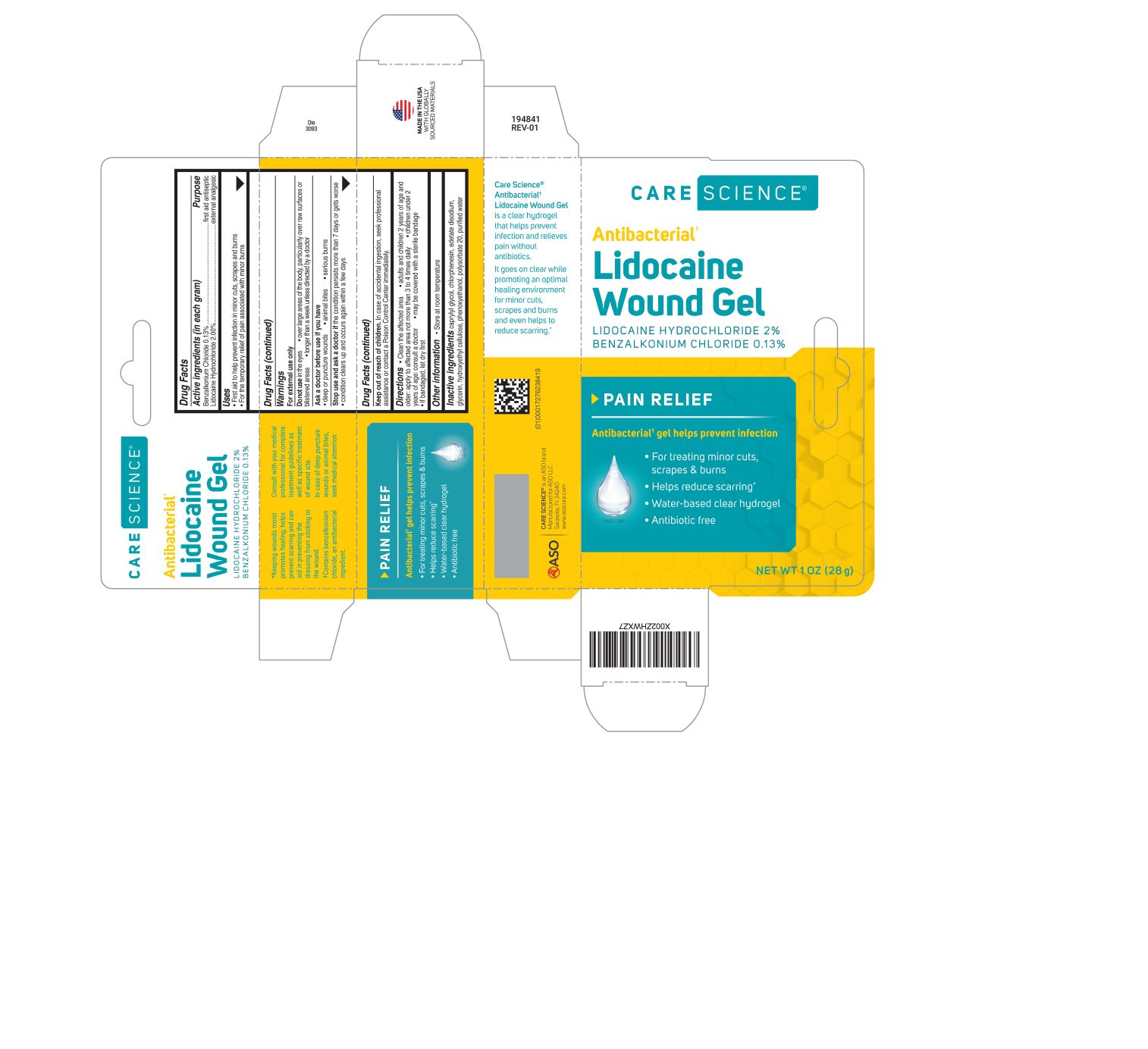
ANTIBACTERIAL LIDOCAINE WOUND GEL- benzalkonium chloride, lidocaine hydrochloride gel
ASO LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each gram)
Benzalkonium Chloride 0.13%
Lidocaine Hydrochloride 2.00%
Purpose
first aid antiseptic
external analgesic
Uses
- First aid to help prevent infection in minor cuts, scrapes and burns
- For the temporary relief of pain associated with minor burns
Warnings
For external use only
Do not use
- in the eyes
- over large areas of the body, particularly over raw surfaces or blistered areas
- longer than a week unless directed by a doctor
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- the condition persists more than 7 days or gets worse
- condition clears up and occurs again within a few days
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions
- clean the affected area
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: consult a doctor
- may be covered with a sterile bandage
- if bandaged, let dry first
Other information
Store at room temperature
Inactive ingredients
caprylyl glycol, chlorphenesin, edetate disodium, glycerin, hydroxyethyl cellulose, phenoxyethanol, polysorbate 20, purified water
Principal Display Panel
CARE SCIENCE
Antibacterial
Lidocaine Wound Gel
Lidocaine Hydrochloride 2%
Benzalkonium Chloride 0.13%
Pain Relief
Antibacterial gel helps prevent infection
For treating minor cuts, scrapes and burns
Helps reduce scarring
Water-based clear hydrogel
Antibiotic free