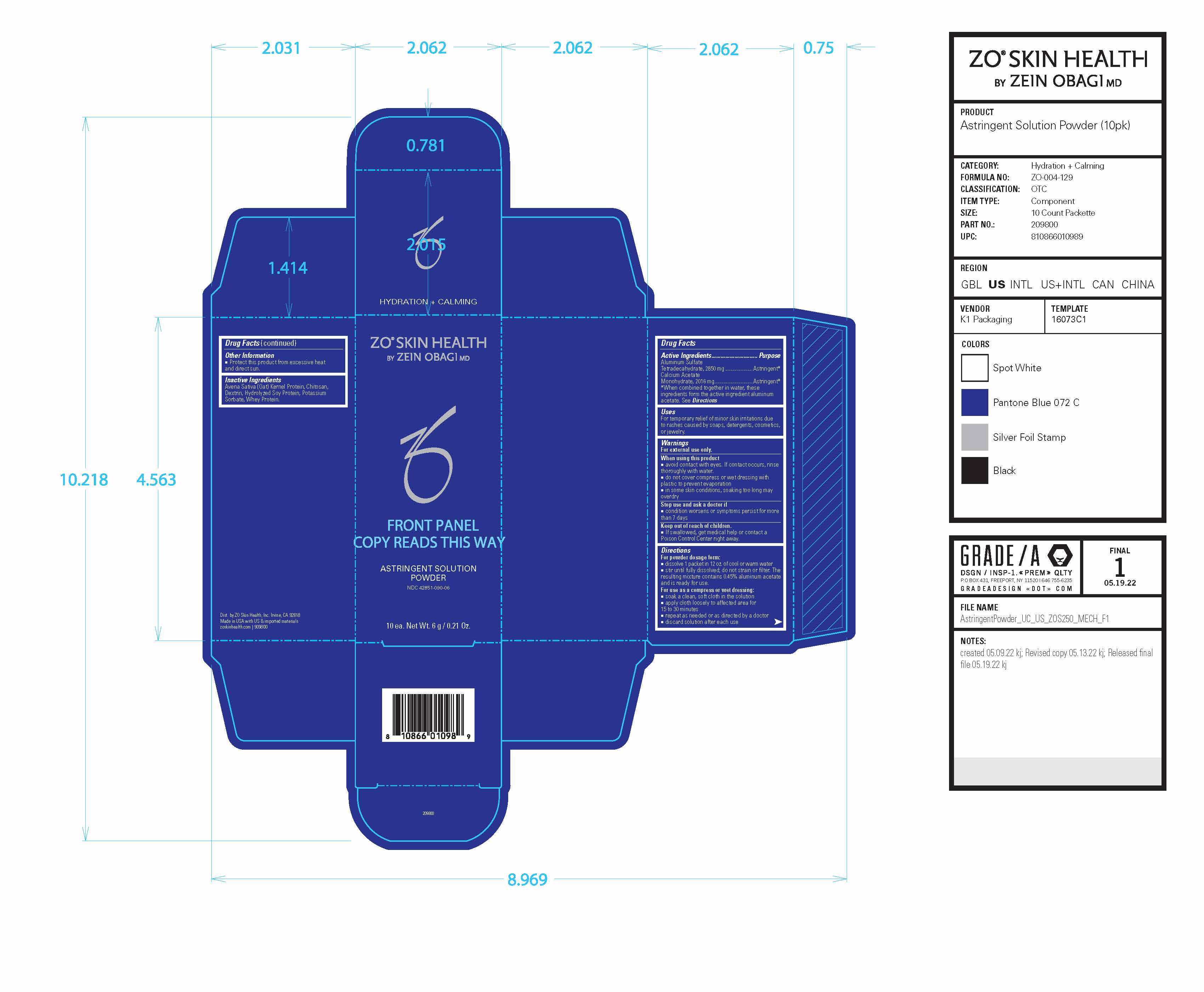
Drug Facts
| Active Ingredients | Purpose |
| Aluminum Sulfate Tetradecahydrate, 2850 mg | Astringent* |
| Calcium AcetateMonohydrate, 2016 mg | Astringent* |
*When combined together in water, these ingredients form the active ingredient aluminum acetate. See Directions
Uses
For temporary relief of minor skin irritations due to rashes caused by soaps, detergents, cosmetics, or jewelry.
Warnings
For external use only.
Directions
For powder dosage form:
■ dissolve 1 packet in 12 oz. of cool or warm water
■ stir until fully dissolved; do not strain or filter. The resulting mixture contains 0.45% aluminum acetate and is ready for use.
For use as a compress or wet dressing:
■ soak a clean, soft cloth in the solution
■ apply cloth loosely to affected area for
15 to 30 minutes
■ repeat as needed or as directed by a doctor
■ discard solution after each use