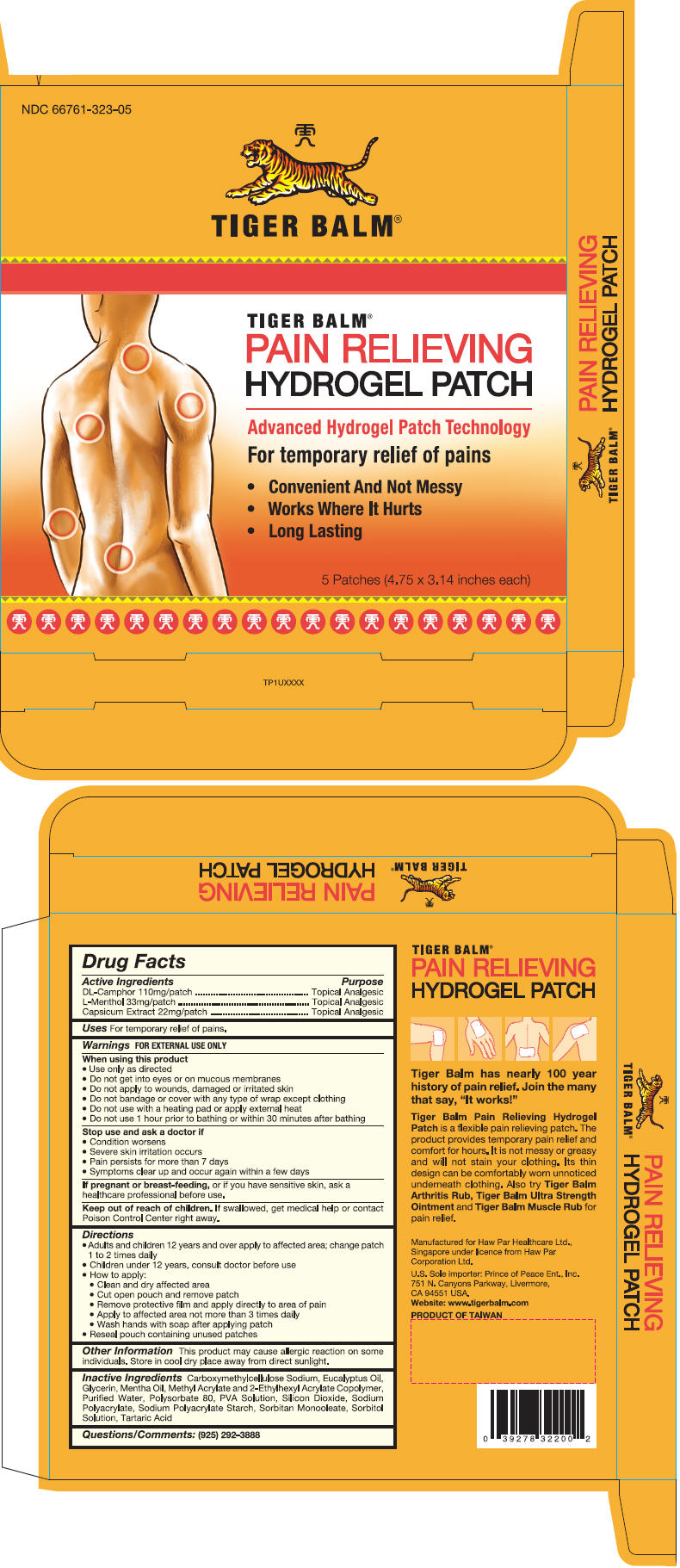
Warnings
FOR EXTERNAL USE ONLY
When using this product
- Use only as directed
- Do not get into eyes or on mucous membranes
- Do not apply to wounds, damaged or irritated skin
- Do not bandage or cover with any type of wrap except clothing
- Do not use with a heating pad or apply external heat
- Do not use 1 hour prior to bathing or within 30 minutes after bathing
Stop use and ask a doctor if
- Condition worsens
- Severe skin irritation occurs
- Pain persists for more than 7 days
- Symptoms clear up and occur again within a few days
Directions
- Adults and children 12 years and over apply to affected area; change patch 1 to 2 times daily
- Children under 12 years, consult doctor before use
- How to apply:
- Clean and dry affected area
- Cut open pouch and remove patch
- Remove protective film and apply directly to area of pain
- Apply to affected area not more than 3 times daily
- Wash hands with soap after applying patch
- Reseal pouch containing unused patches
Other Information
This product may cause allergic reaction on some individuals. Store in cool dry place away from direct sunlight.
Inactive Ingredients
Carboxymethylcellulose Sodium, Eucalyptus Oil, Glycerin, Mentha Oil, Methyl Acrylate and 2-Ethylhexyl Acrylate Copolymer, Purified Water, Polysorbate 80, PVA Solution, Silicon Dioxide, Sodium Polyacrylate, Sodium Polyacrylate Starch, Sorbitan Monooleate, Sorbitol Solution, Tartaric Acid