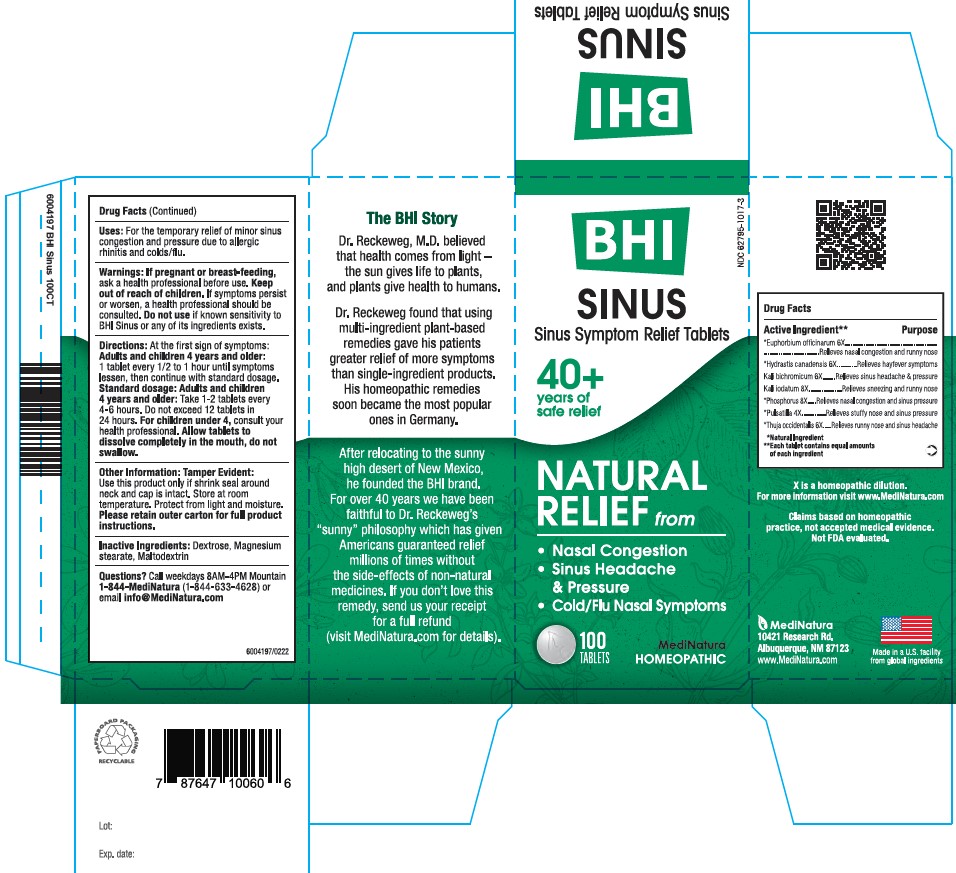
ACTIVE INGREDIENTS
Each tablet contains: *Euphorbium officinarum 6X, *Hydrastis canadensis 6X, Kali bichromicum 6X, Kali iodatum 8X, *Phosphorus 8X, *Pulsatilla 4X, *Thuja occidentalis 6X 42.9 mg each.
*Natural Ingredients
PURPOSE
Sinus Relief Tablets
Relieves:
• Nasal Congestion
• Sinus Headache & Pressure
• Cold/Flu Nasal Symptoms
USES
For the temporary relief of minor sinus congestion and pressure due to allergic rhinitis and colds
Directions
At first sign of symptoms: Adults and children 4 years and older: 1 tablet every 1/2 to 1 hour until symptoms lessen, then continue with standard dosage.
Standard dosage: Adults and children 4 years and older: Take 1-2 tablet every 4 to 6 hours. Do not exceed 12 tablets in 24 hours. For children under 4, consult your health professional.
Allow tablets to dissolve completely in the mouth, do not swallow.