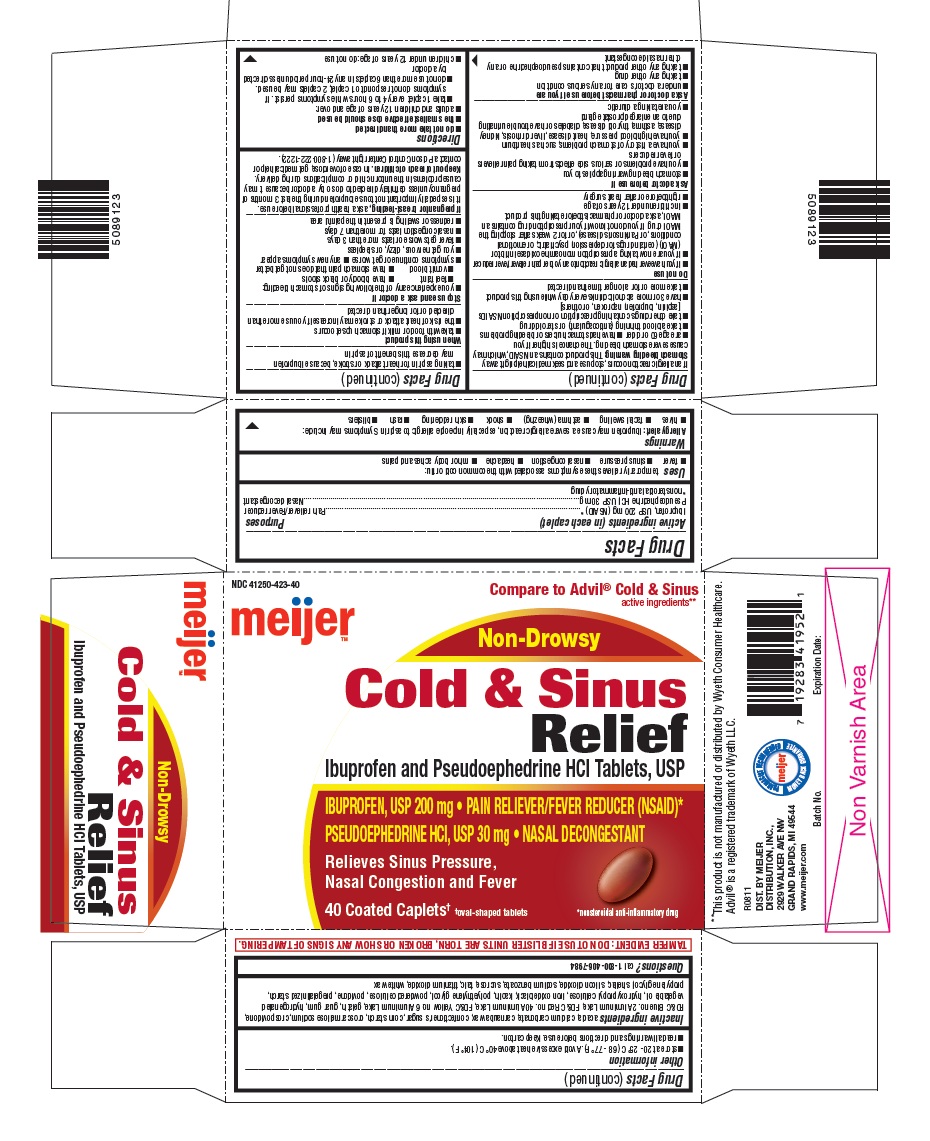
USES
Temporarily relieves these symptoms associated with the common cold or flu:
- fever
- sinus pressure
- nasal congestion
- headache
- minor body aches and pains
WARNINGS
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- in children under 12 years of age
- right before or after heart surgery
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have problems or serious side effects from taking pain relievers or fever reducers
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, thyroid disease, diabetes or have trouble urinating due to an enlarged prostate gland
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
- under a doctor’s care for any serious condition
- taking any other drug
- taking any other product that contains pseudoephedrine or any other nasal decongestant
- taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
When using this product
- take with food or milk if stomach upset occurs
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- have bloody or black stools
- vomit blood
- have stomach pain that does not get better
- symptoms continue or get worse
- any new symptoms appear
- you get nervous, dizzy, or sleepless
- fever gets worse or lasts more than 3 days
- nasal congestion lasts for more than 7 days
- redness or swelling is present in the painful area
DIRECTIONS
- do not take more than directed
- the smallest effective dose should be used
- adults and children 12 years of age and over:
- take 1 caplet every 4 to 6 hours while symptoms persist. If symptoms do not respond to 1 caplet, 2 caplets may be used.
- do not use more than 6 caplets in any 24-hour period unless directed by a doctor
- children under 12 years of age: do not use
OTHER INFORMATION
- store at 20 - 25○ C (68 - 77○ F). Avoid excessive heat above 40○ C (104○ F).
- read all warnings and directions before use. Keep carton.
- TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
INACTIVE INGREDIENTS
Acacia, calcium carbonate, carnauba wax, confectioner’s sugar, corn starch, croscarmellose sodium, crospovidone, FD&C Blue no. 2 Aluminum Lake, FD&C Red no. 40 Aluminum Lake, FD&C Yellow no. 6 Aluminum Lake, gelatin, guar gum, hydrogenated vegetable oil, hydroxypropyl cellulose, iron oxide black, kaolin, polyethylene glycol, powdered cellulose, povidone, pregelatinized starch, propylene glycol, shellac, silicon dioxide, sodium benzoate, sucrose, talc, titanium dioxide, white wax
PRINCIPAL DISPLAY PANEL
Compare to Advil®Cold & Sinus active ingredients**
Ibuprofen and Pseudoephedrine HCl Tablets, USP
IBUPROFEN, USP 200 mg · PAIN RELIEVER/FEVER REDUCER (NSAID)*
PSEUDOEPHEDRINE HCl, USP 30 mg · NASAL DECONGESTANT
Relieves Sinus Pressure, Nasal Congestion and Fever