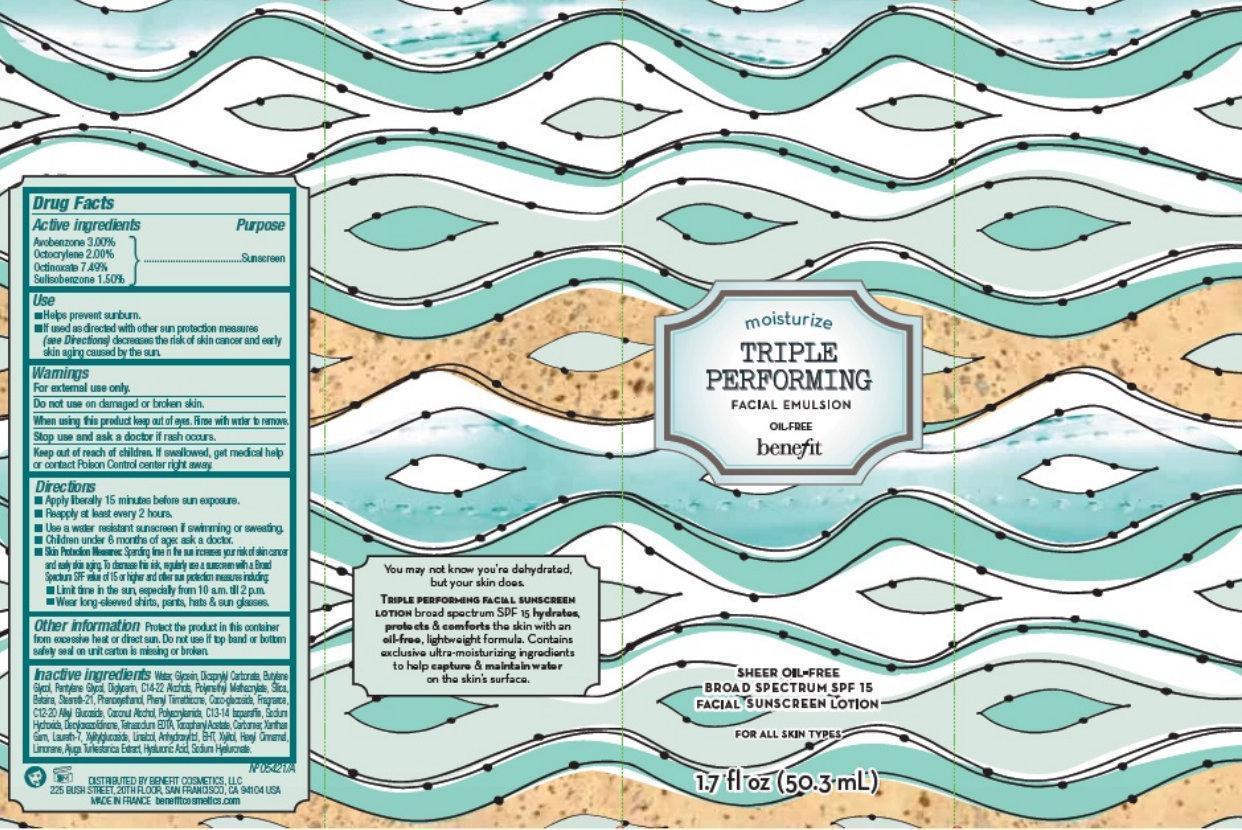
BENEFIT TRIPLE PERFORMING FACIAL EMULSION BROAD SPECTRUM SPF 15- avobenzone, octocrylene, octinoxate, sulisobenzone emulsion
Benefit Cosmetics, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Benefit Triple Performing Facial Emulsion Broad Spectrum SPF 15
Use
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see
Directions) decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating.
- Children under 6 months of age: ask a doctor
- Skin Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially 10 a.m. till 2 p.m.
- Wear long-sleeved shirts, hats, and sunglasses.
Other information
Protect the product in this container from excessive heat or direct sun. Do not use if top band and bottom safety seal on unit carton is missing or broken.
Inactive ingredients
Water, Glycerin, Dicaprylyl Carbonate, Butylene Glycol, Pentylene Glycol, Diglycerin, C14-22 Alcohols, Polymethyl Methacrylate, Silica, Bataira, Steareth-21, Phenoxyethanol, Phenyl Trimethicone, Coco-glucoside, Fragrance, C12-20 Alkyl Glucoside, Coconut Alcohol, Polyacrylamide, C13-14 Isoparaffin, Sodium Hydroxide, De, Tetrasodium EDTA, Tocopheryl Acetate, Carbomer, Xanthan Gum, Laureth-7, Xylitylglucoside, Linalool, Anhydroxylitol, BHT, Xyltol, Hexyl Cinnamal, Linoate, Augi Tulestica Extract, Hyaluronic Acid, Sodium Hyaluronate.
You may not know you're dehydrated, but your skin does.
TRIPLE PERFORMING FACIAL SUNSCREEN LOTION broad spectrum SPF 15 hydrates, protects and comforts the skin with an oil-free, lightweight formula. Contains exclusive ultra-moisturizing ingredients to help capture and maintain water on the skin's surface. DISTRIBUTED BY BENEFIT COSMETICS, LLC 225 BUSH STREET, 20TH FLOOR, SAN FRANCISCO, CA 94104 USA MADE IN FRANCE benefitcosmetics.com
| BENEFIT TRIPLE PERFORMING FACIAL EMULSION BROAD SPECTRUM SPF 15
avobenzone, octocrylene, octinoxate, sulisobenzone emulsion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Benefit Cosmetics, LLC (070826813) |