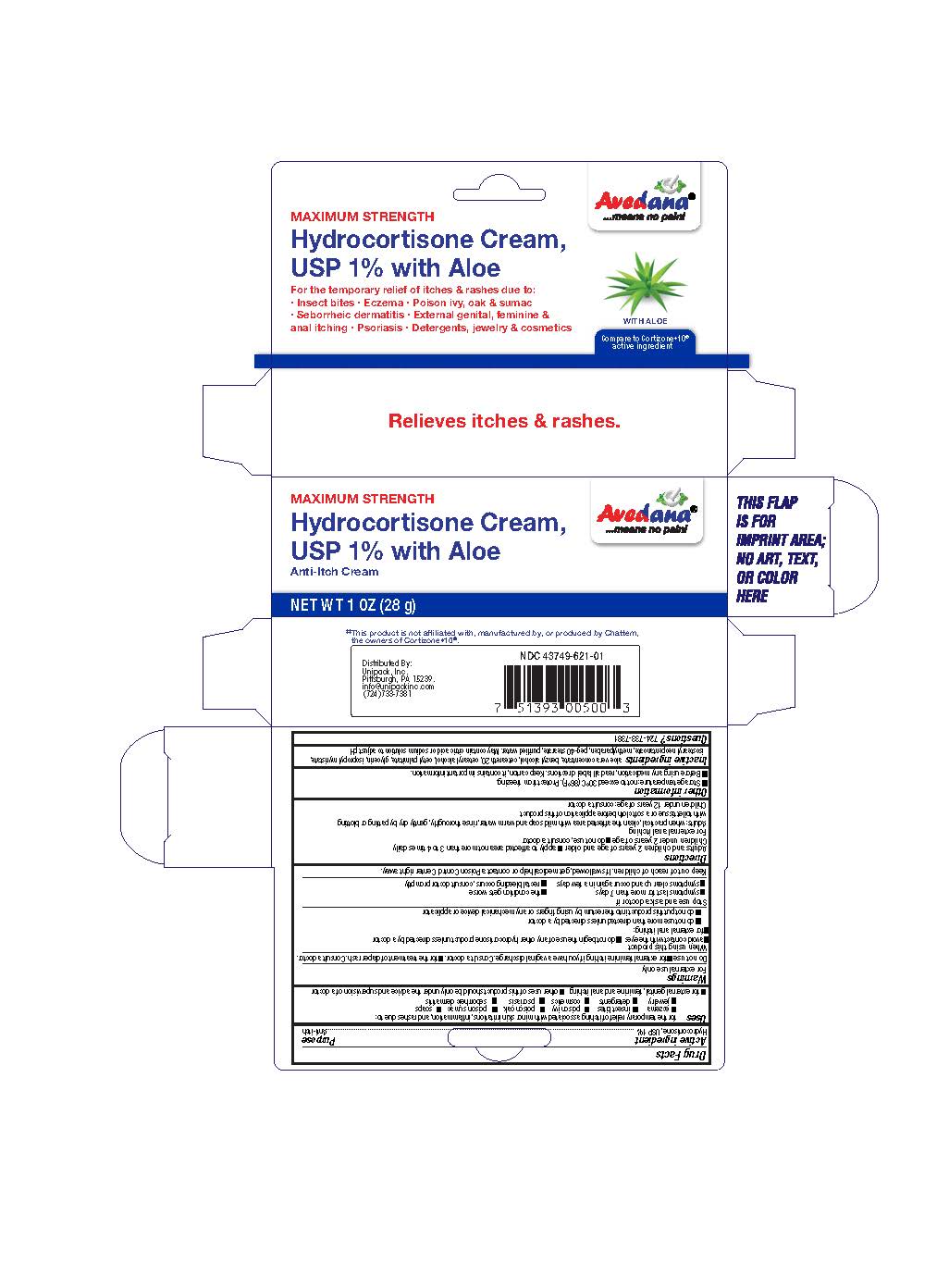
Uses
for the temporary relief of itching associated with minor skin irritations, inflammation, and rashes due to:
- eczema
- insect bites
- poison ivy
- poison oak
- poison sumac
- soaps
- jewelry
- detergents
- cosmetics
- psoriasis
- seborrheic dermatitis
- for external gential, feminine and anal itching
- other uses of this product should be only under the advice and supervision of a doctor
Do not use
- for external feminine itching if you have a vaginal discharge. Consult a doctor.
- for the treatment of diaper rash. Consult a doctor.
When using this product
- avoid contact with the eyes
- do not begin the use of any other hydrocortisone product unless directed by a doctor
- for external anal itching
- do not use more than directed unless directed by a doctor
- do not put this product into the rectum by using fingers or any mechanical device or applicator
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 2 years of age and older * apply to affected area not more than 3 to 4 times daily
Children under 2 years of age *do not use, consult a doctor
For external anal itching
Adults: when practical, clean the affected area with mild soap and warm water, rinse thoroughly, gently dry by patting or blotting with toilet tissue or a solft cloth before application of this product.
Children under 12 years of age: consult a doctor
Other information
- Storage temperature: not to exceed 30 °C (86 °F). Protect from freezing.
- Before using any medication, read all label directions. Keep carton, it contains important information.