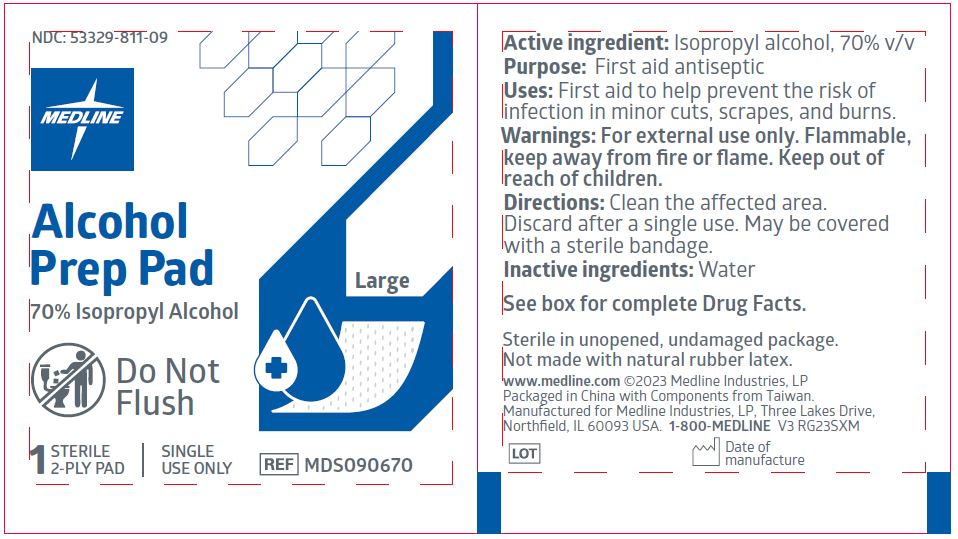
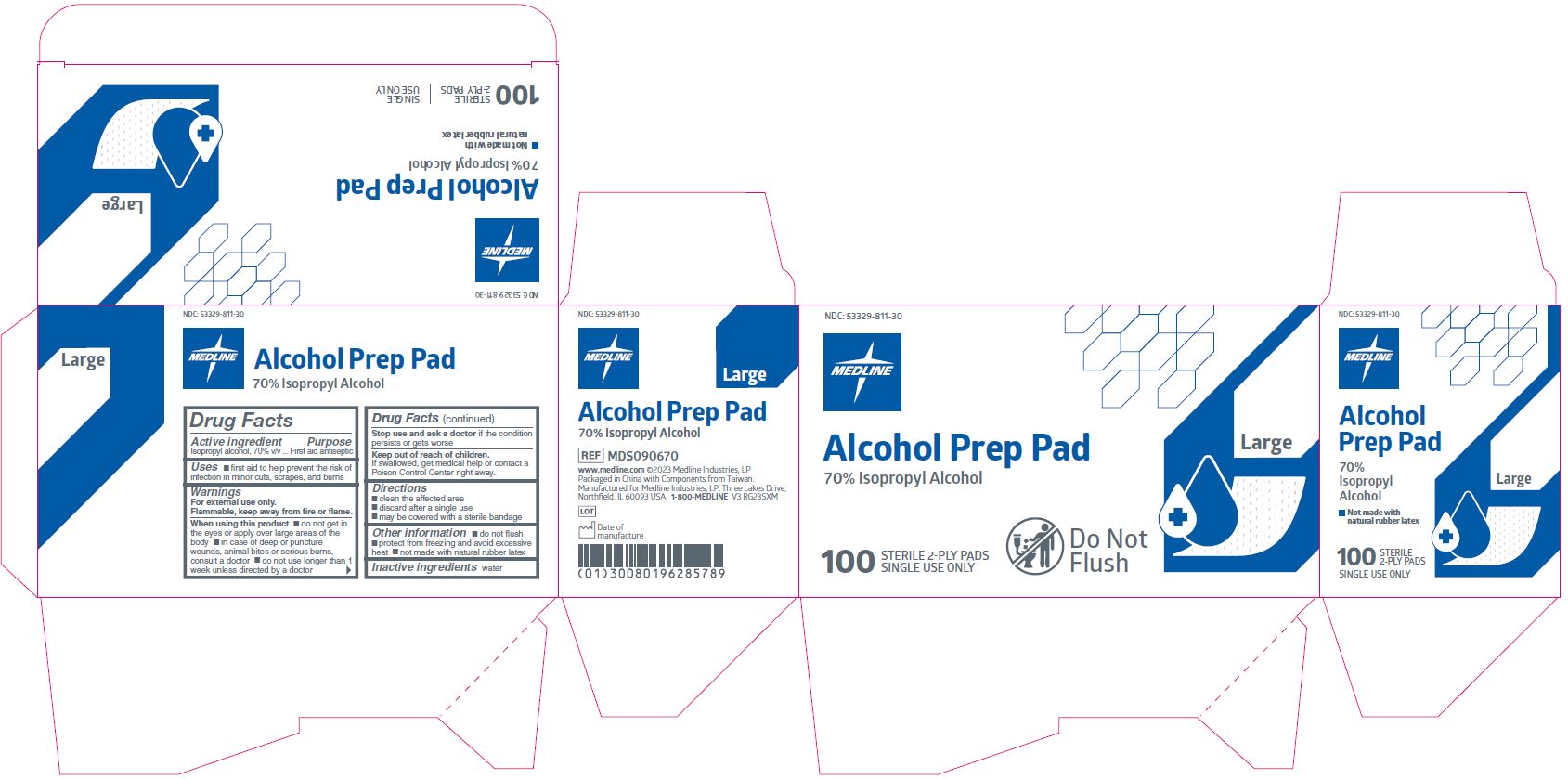
MEDLINE ALCOHOL PREP STERILE, LARGE- isopropyl alcohol swab
Medline Industries, LP
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Isopropyl alcohol 70% v/v
Purpose
First aid antiseptic
Uses
- first aid to help prevent the risk of infection in minor cuts, scrapes, and burns
Warnings
For external use only.
Flammable, keep away from fire or flame.
When using this product
- do not get in the eyes or apply over large areas of the body
- in case of deep or puncture wounds, animal bites or serious burns, consult a doctor
- do not use longer than 1 week unless directed by a doctor
Stop use and ask a doctor if
- condition persists or gets worse
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean the affected area
- discard after a single use
- may be covered with a sterile bandage
Other information
- protect from freezing, avoid excessive heat
- do not flush
- not made with natural rubber latex
Inactive ingredient
water
Manufacturing Information
Manufactured for:
Medline Industries, LP
Three Lakes Drive, Northfield, IL 60093 USA
Packaged in China with Components from Taiwan
www.medline.com
1-800-MEDLINE (633-5463)
REF: MDS090670
V3 RG23SXM
Package Label