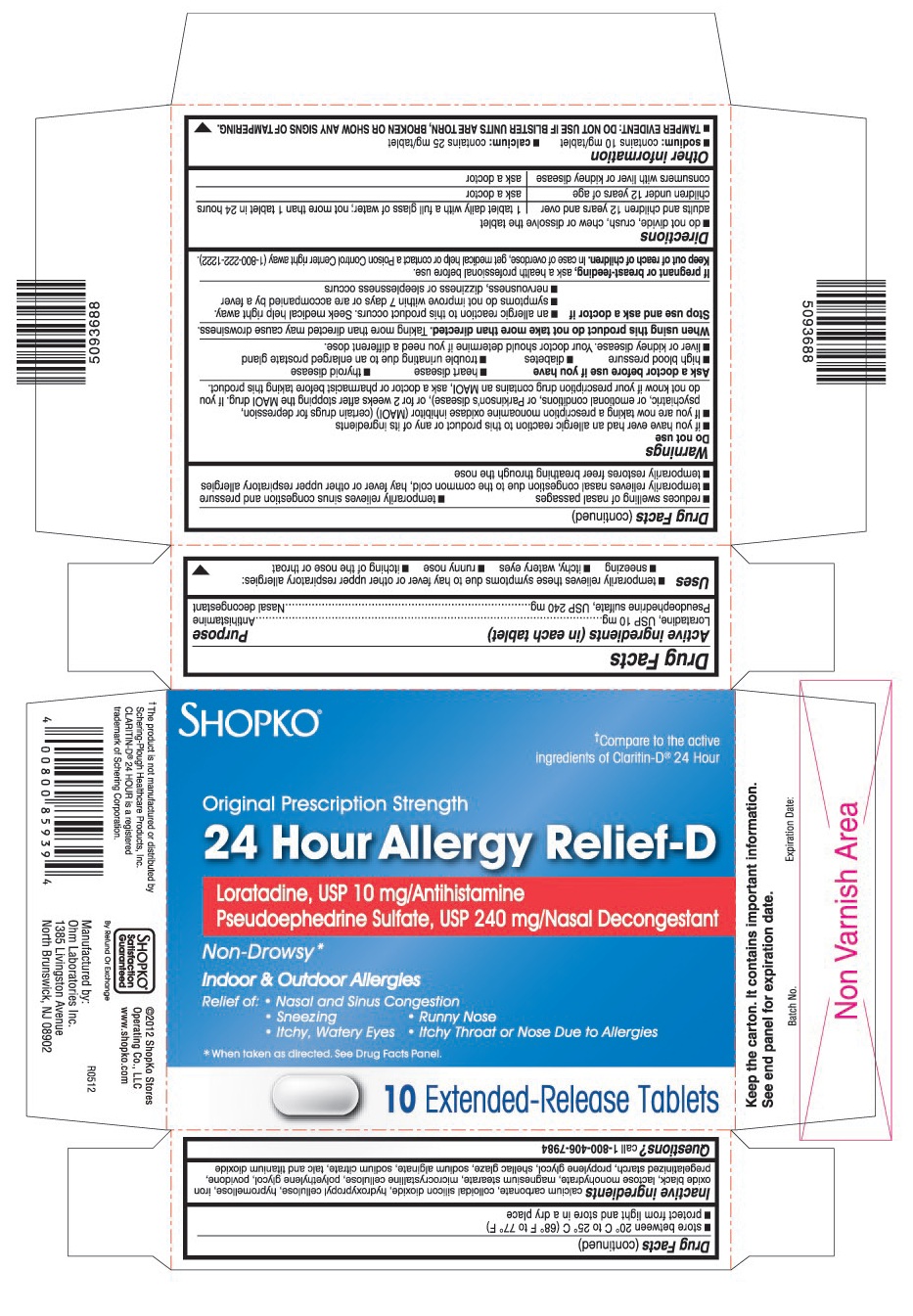
USES
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- sneezing
- itchy, watery eyes
- runny nose
- itching of the nose or throat
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- temporarily restores freer breathing through the nose
WARNINGS
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- thyroid disease
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate gland
- liver or kidney disease. Your doctor should determine if you need a different dose.
OTHER INFORMATION
- sodium: contains 10 mg/tablet
- calcium: contains 25 mg/tablet
- TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
- store between 20° C to 25° C (68° F to 77° F).
- protect from light and store in a dry place
INACTIVE INGREDIENTS
Calcium carbonate, colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose, iron oxide black, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, pregelatinized starch, propylene glycol, shellac glaze, sodium alginate, sodium citrate, talc and titanium dioxide
PRINCIPAL DISPLAY PANEL
†Compare to the active ingredients of Claritin-D®24 Hour
Original Prescription Strength
Loratadine, USP 10 mg/Antihistamine
Pseudoephedrine Sulfate, USP 240 mg/Nasal Decongestant
- Nasal and Sinus Congestion
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose Due to Allergies
*When taken as directed. See Drug Facts Panel.