Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Directions
- take every 4 to 6 hours, or as directed by a doctor
| adults and children 12 years and over | 1 tablet. Do not exceed 6 tablets in 24 hours. |
| children 6 to under 12 years | 1/2 tablet (break tablet in half). Do not exceed 3 whole tablets in 24 hours. |
| children under 6 years | do not use |
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- protect from excessive moisture
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, D&C yellow #10 aluminum lake, lactose anhydrous, magnesium stearate, microcrystalline cellulose
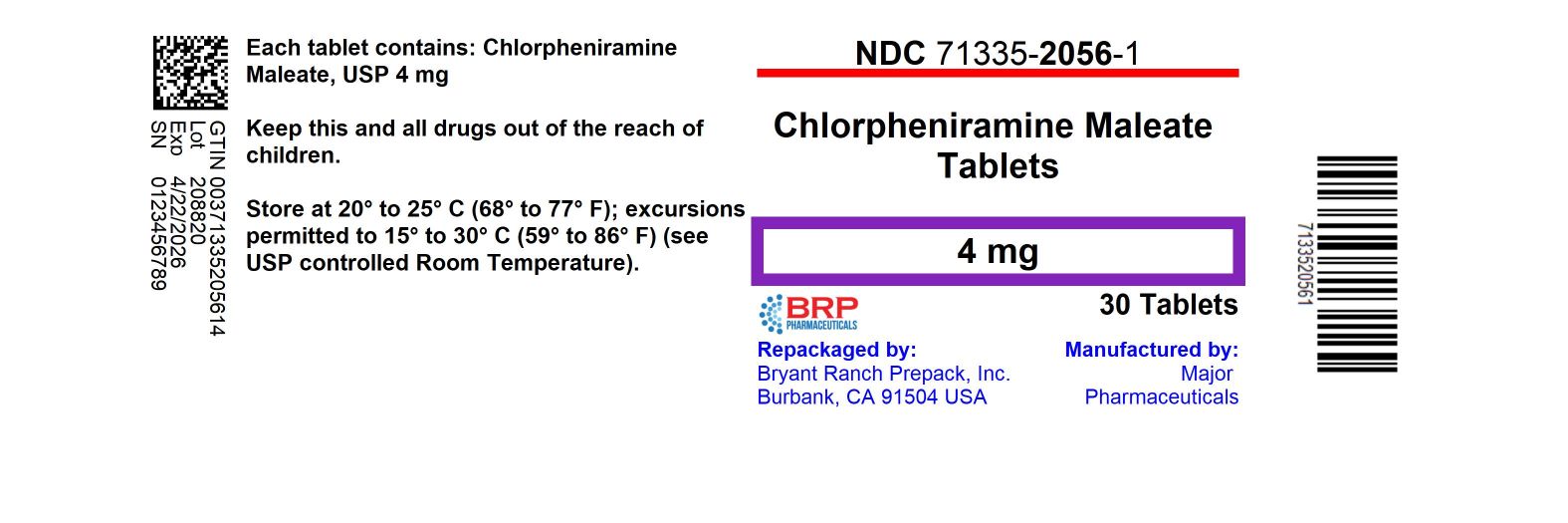
HOW SUPPLIED
Chlorpheniramine Maleate 4 mg
NDC: 71335-2056-1: 30 Tablets in a BOTTLE, PLASTIC
NDC: 71335-2056-2: 60 Tablets in a BOTTLE, PLASTIC
NDC: 71335-2056-3: 100 Tablets in a BOTTLE, PLASTIC
NDC: 71335-2056-4: 120 Tablets in a BOTTLE, PLASTIC
NDC: 71335-2056-5: 40 Tablets in a BOTTLE, PLASTIC
NDC: 71335-2056-6: 24 Tablets in a BOTTLE, PLASTIC
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504