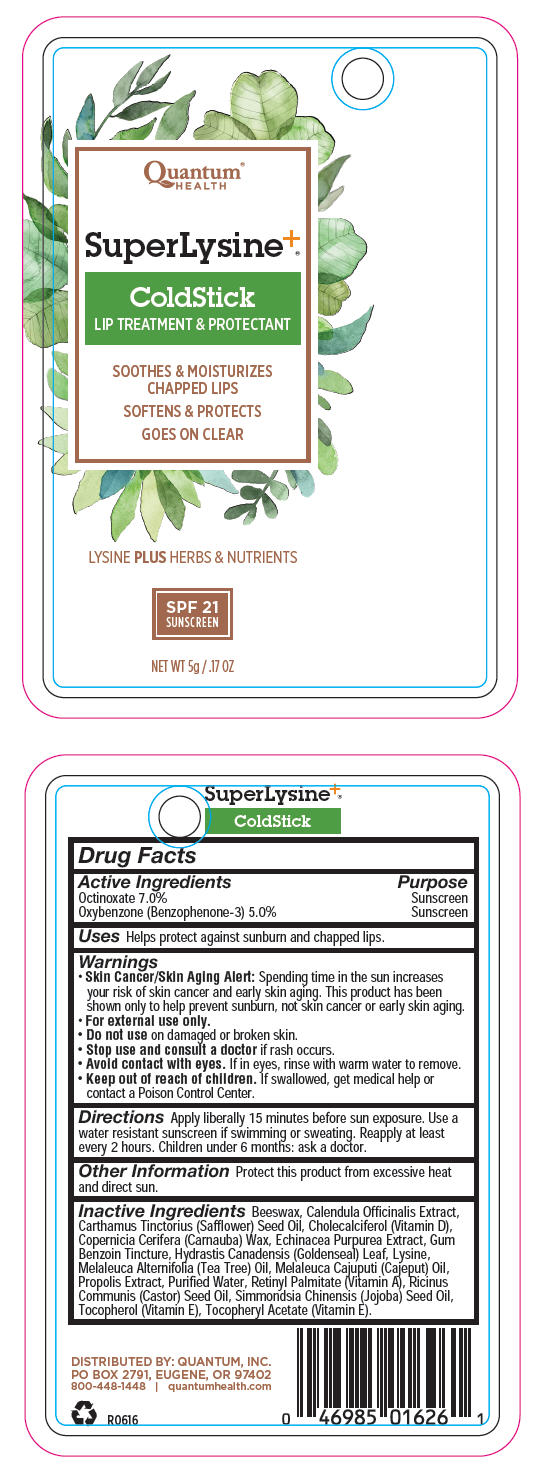
SUPER LYSINE PLUS COLDSTICK- octinoxate and oxybenzone lipstick
Quantum, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| Active Ingredients | Purpose |
| Octinoxate 7.0% | Sunscreen |
| Oxybenzone (Benzophenone-3) 5.0% | Sunscreen |
Uses
Helps protect against sunburn and chapped lips.
Warnings
-
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
-
For external use only.
-
Do not use on damaged or broken skin.
-
Stop use and consult a doctor if rash occurs.
-
Avoid contact with eyes. If in eyes, rinse with warm water to remove.
-
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center.
Directions
Apply liberally 15 minutes before sun exposure. Use a water resistant sunscreen if swimming or sweating. Reapply at least every 2 hours. Children under 6 months: ask a doctor.
Other Information
Protect this product from excessive heat and direct sun.
Inactive Ingredients
Beeswax, Calendula Officinalis Extract, Carthamus Tinctorius (Safflower) Seed Oil, Cholecalciferol (Vitamin D), Copernicia Cerifera (Carnauba) Wax, Echinacea Purpurea Extract, Gum Benzoin Tincture, Hydrastis Canadensis (Goldenseal) Leaf, Lysine, Melaleuca Alternifolia (Tea Tree) Oil, Melaleuca Cajuputi (Cajeput) Oil, Propolis Extract, Purified Water, Retinyl Palmitate (Vitamin A), Ricinus Communis (Castor) Seed Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Tocopherol (Vitamin E), Tocopheryl Acetate (Vitamin E).
DISTRIBUTED BY: QUANTUM, INC.
PO BOX 2791, EUGENE, OR 97402
PRINCIPAL DISPLAY PANEL - 5 g Cylinder Blister Pack
Quantum®
HEALTH
SuperLysine+®
ColdStick
LIP TREATMENT & PROTECTANT
SOOTHES & MOISTURIZES
CHAPPED LIPS
SOFTENS & PROTECTS
GOES ON CLEAR
LYSINE PLUS HERBS & NUTRIENTS
SPF 21
SUNSCREEN
NET WT 5g / .17 OZ
Quantum, Inc.