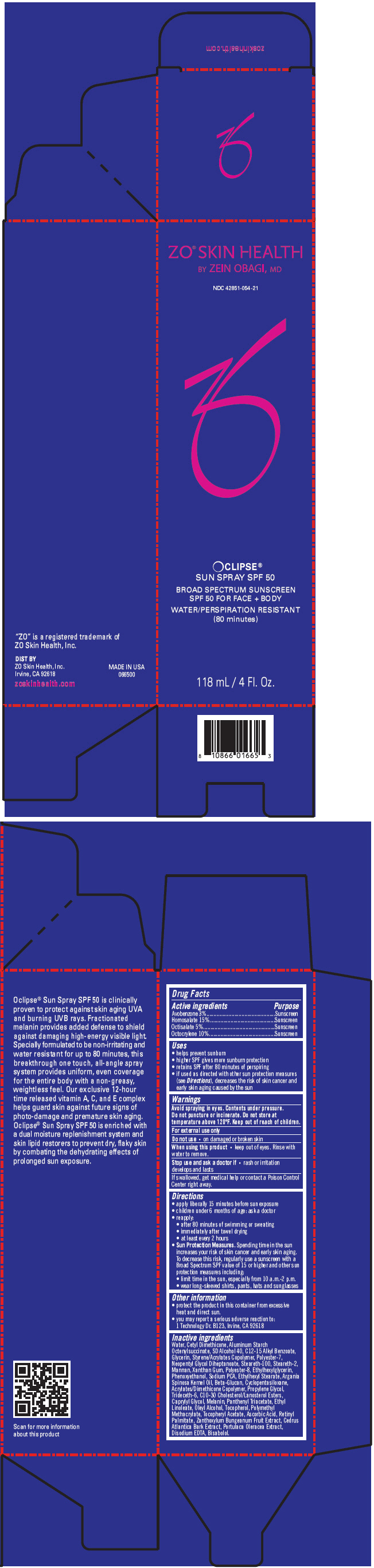
ZO SKIN HEALTH OCLIPSE SUN SPF 50 BROAD SPECTRUM SUNSCREEN SPF 50 FOR FACE PLUS BODY WATER/PERSPIRATION RESISTANT (80 MINUTES)- avobenzone, homosalate, octisalate, and octocrylene aerosol, spray
ZO Skin Health, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| Active ingredients | Purpose |
| Avobenzone 3% | Sunscreen |
| Homosalate 15% | Sunscreen |
| Octisalate 5% | Sunscreen |
| Octocrylene 10% | Sunscreen |
Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
- retains SPF after 80 minutes of perspiring
- if used as directed with other sun protection measures (see
Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
Avoid spraying in eyes. Contents under pressure. Do not puncture or incinerate. Do not store at temperature above 120ºF.
Keep out of reach of children.
Do not use
- on damaged or broken skin
When using this product
- keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- rash or irritation develops and lasts
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
- children under 6 months of age: ask a doctor
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
Other information
- protect the product in this container from excessive heat and direct sun.
- you may report a serious adverse reaction to:
1 Technology Dr. B123, Irvine, CA 92618
Inactive ingredients
Water, Cetyl Dimethicone, Aluminum Starch Octenylsuccinate, SD Alcohol 40, C12-15 Alkyl Benzoate, Glycerin, Styrene/Acrylates Copolymer, Polyester-7, Neopentyl Glycol Diheptanoate, Steareth-100, Steareth-2, Mannan, Xanthan Gum, Polyester-8, Ethylhexylglycerin, Phenoxyethanol, Sodium PCA, Ethylhexyl Stearate, Argania Spinosa Kernel Oil, Beta-Glucan, Cyclopentasiloxane, Acrylates/Dimethicone Copolymer, Propylene Glycol, Trideceth-6, C10-30 Cholesterol/Lanosterol Esters, Caprylyl Glycol, Melanin, Panthenyl Triacetate, Ethyl Linoleate, Oleyl Alcohol, Tocopherol, Polymethyl Methacrylate, Tocopheryl Acetate, Ascorbic Acid, Retinyl Palmitate, Zanthoxylum Bungeanum Fruit Extract, Cedrus Atlantica Bark Extract, Portulaca Oleracea Extract, Disodium EDTA, Bisabolol.
DIST BY
ZO Skin Health, Inc.
Irvine, CA 92618
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Carton
ZO
® SKIN HEALTH
BY ZEIN OBAGI, MD
NDC 42851-054-21
OCLIPSE
®
SUN SPRAY SPF 50
BROAD SPECTRUM SUNSCREEN
SPF 50 FOR FACE + BODY
WATER/PERSPIRATION RESISTANT
(80 minutes)
118 mL / 4 Fl. Oz.
ZO Skin Health, Inc.