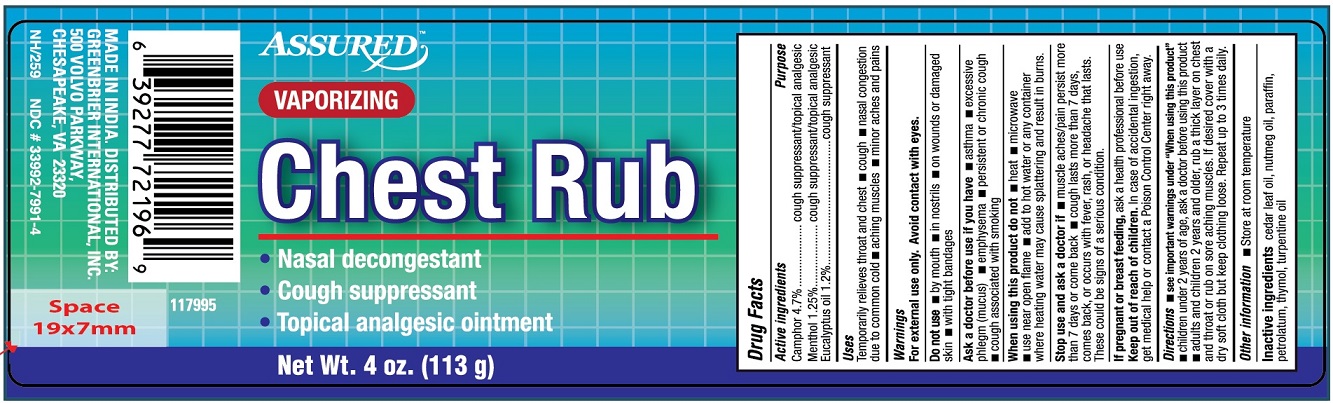
Uses
Temporarily relieves throat and chest
- cough
- nasal congestion due to common cold
- aching muscles
- minor aches and pains
Ask a doctor before use if you have
- asthama
- excessive phlegm (mucus)
- emphysema
- persistent or chronic cough
- cough associated with smoking
When using this product do not
- heat
- microwave
- use near open flame
- add to hot water or any container where heating water may cause splattering and result in burns
Stop use and ask a doctor if
- muscle aches/pain persist more than 7 days or come back
- cough lasts more than 7 days, comes back, or occurs with fever, rash or headache that lasts. These could be signs of a serious conditions.
Keep out of reach of children. In case of accidental ingestion, get medical help or contact a Poison Control Centre right away.
Directions
- see important warnings under "When using this product"
- children under 2 years of age, ask a doctor before using this product
- adults and children 2 years and older, rub a thick layer on chest and throat or rub on sore aching muscles. If desired, cover with a dry soft cloth but keep clothing loose. Repeat up to 3 times daily.