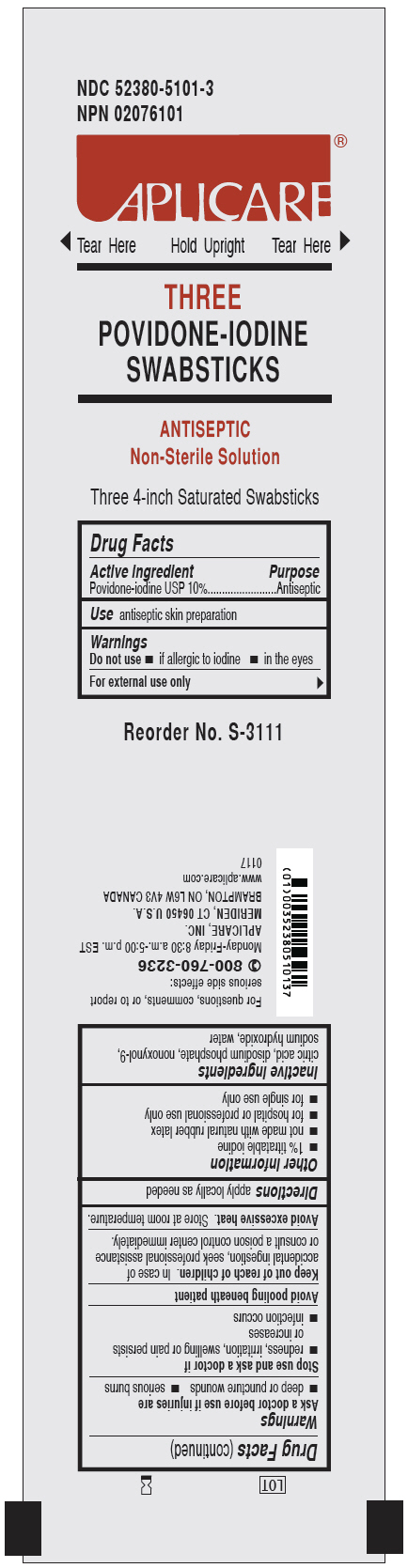
APLICARE POVIDONE-IODINE TRIPLES- povidone-iodine solution
Aplicare Products, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Povidone-iodine USP 10%
Use
antiseptic skin preparation
Warnings
-
For external use only
-
Avoid pooling beneath patient
Do not use
- • if allergic to iodine
- • in the eyes
Ask a doctor before use if injuries are
- • deep or puncture wounds
- • serious burns
Stop use and ask a doctor if
- • redness, irritation, swelling or pain persists or increases
- • infection occurs
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or consult a poison control center immediately.
Storage Information
Avoid excessive heat.Store at room temperature.
Other information
- • 1% titratable iodine
- • not made with natural rubber latex
- • for hospital or professional use only
- • for single use only
Inactive ingredients
citric acid, disodium phosphate, nonoxynol-9, sodium hydroxide, water
Manufacturing Information
Manufactured by:
Aplicare Products, LLC
550 Research Parkway, Meriden, CT 06450 USA
Manufactured for:
Medline Industries, LP
Three Lakes Drive, Northfield, IL 60093 USA
Made in USA with domestic and foreign materials
1-800-633-5463
REF: APLS3111
0117
Package Label