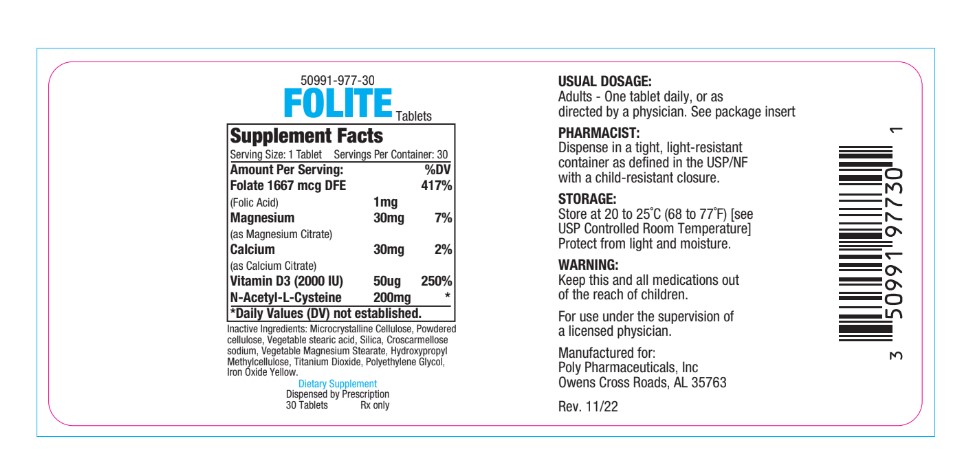
Indication and Usage
Folite is indicated for folic acid supplementation and general nutritional support. May also be recommended for patients who are of advancing
age or suffer from improper food intake.
Precaution
Folic Acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive.
Warnings
KEEP THIS AN ALL MEDICATION OUT OF REACH OF CHILDREN.
For use under the supervision of a licensed physician. Dispense in a tight, light resistant container as defined in the USP/NF with a child-resistant closure.
Storage
Store at 20 to 25°C (68 to 77°F) [see USP Controlled Room Temperature|
Protect from light and moisture.