Active ingredients:
Arsenicum album 12X, Calcarea carbonica 30X, Kali bichromicum 12X, Mercurius solubilis 30X, Mercurius sulphuratus ruber 12X, Pulsatilla 12X, Sepia 12X, Sulphur 30X, 1 g each in 10 g.
Uses
For the temporary relief of minor symptoms associated with sinusitis such as:
- Sinus headache
- Nasal congestion
- Sinus pressure and pain
- Runny nose
- Sneezing
Warnings
When using this product
- in rare cases, hypersalivation may occur after use, in which case the use of the product should be discontinued
- in very rare cases, skin reactions may occur after use, in which case the product should be discontinued.
Directions
Adults and children ≥ 12 years 5-10 drops 1-3 times daily, acute cases 4-6 times daily in a little water or undiluted or as recommended.
Not recommended for children under 12 years of age except under the advice of a health care professional.
Other information
Tamper resistant: do not use if safety ring at base of cap is broken.
Retain outer carton for full product instructions.
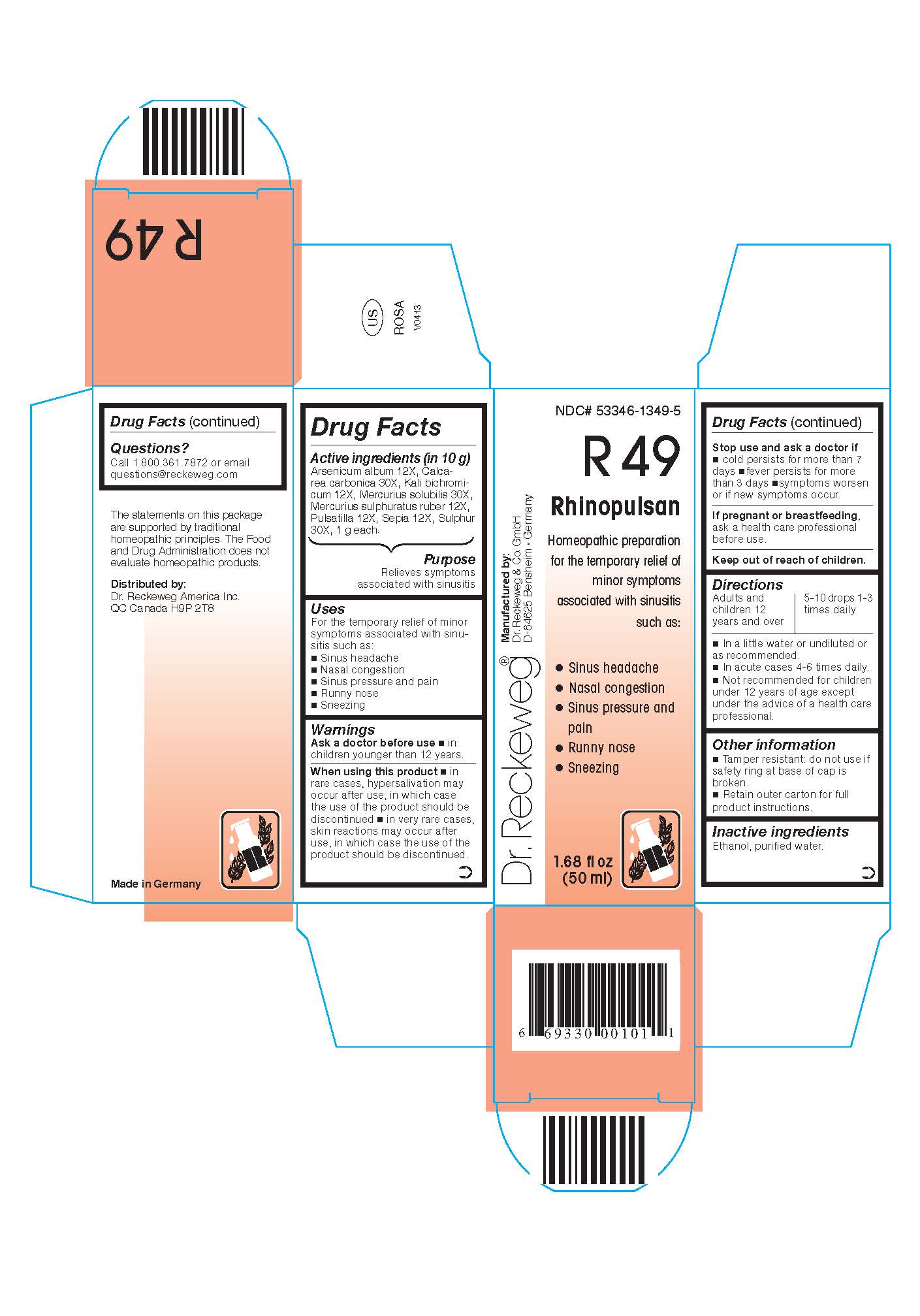 NDC# 53346-1349-5
NDC# 53346-1349-5
Dr. Reckeweg R49 Rhinopulsan
Homeopathic preparation for the temporary relief of minor symptoms associated with sinusitis such as:
- Sinus headache
- Nasal congestion
- Sinus pressure and pain
- Runny nose
- Sneezing
Manufactured by:
Dr. Reckeweg Co. GmbH
D-64625 Bensheim
Germany
1.68 fl oz
(50 ml)