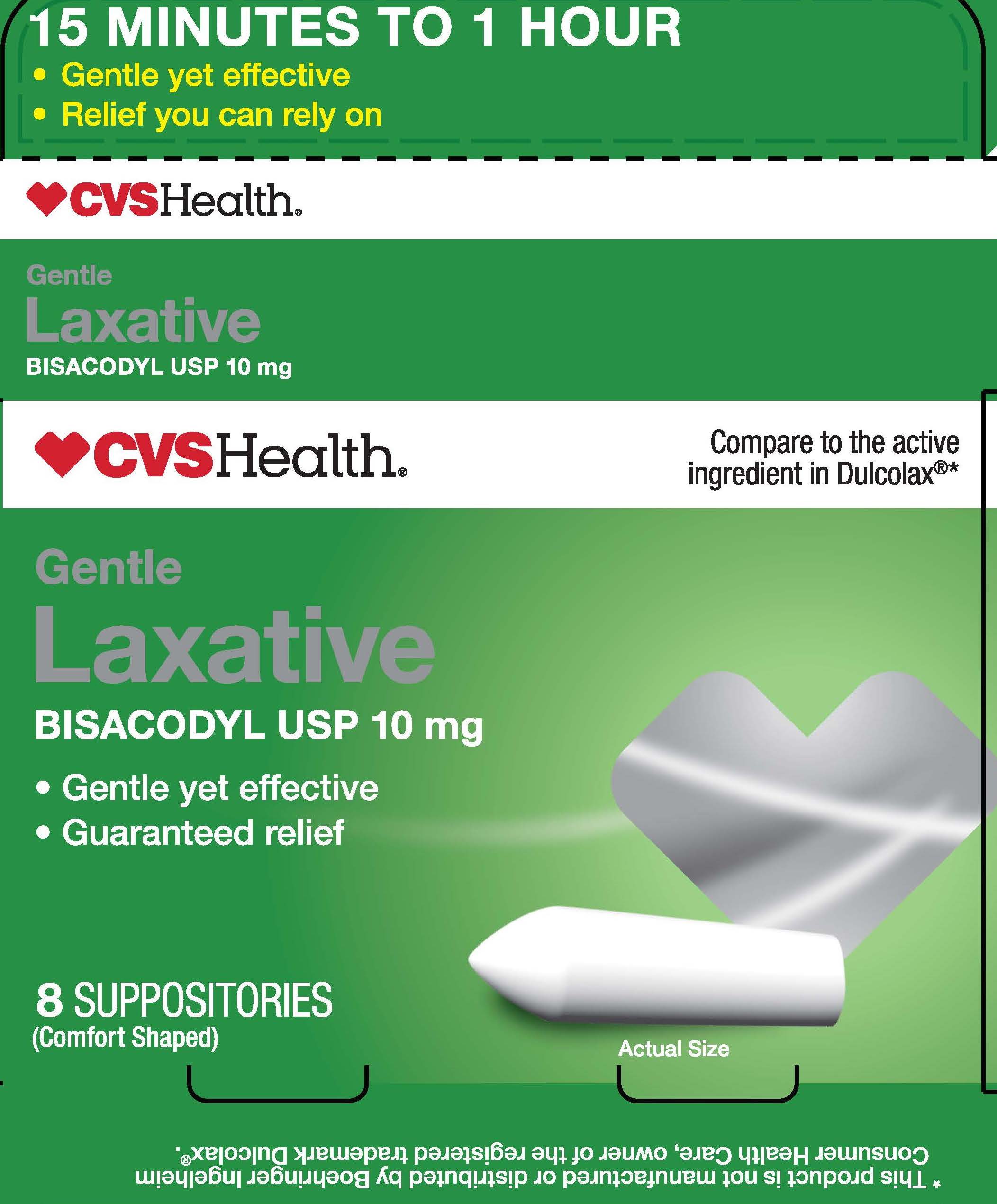
Active ingredient (in each suppository)
Bisacodyl USP 10mg
Purpose
Stimulant Laxative
Use
- for temporary relief of occasional constipation
- this product generally produces bowel movement in 15 minutes to 1 hour
Warnings
For rectal use only
Do not use unless directed by a doctor
- if abdominal pain, nausea or vomiting are present
- for longer than 1 week
Ask a doctor before use if you have
noticed a sudden change in bowel habits that last over a period 2 weeks
When using this product
it may cause stomach discomfort, faintness, rectal burning and mild cramps
Stop use and ask a doctor if
you have rectal bleeding or fail to have a bowel movement after using this product. These may indicate a serious condition.
If pregnant or breast feeding,
ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- detach one suppository from the strip
- remove wrapper berore inserting into rectum
adults and children 12 years of age and over - 1 suppository once daily
children 6 to under 12 years of age - 1/2 suppository once daily
children under 6 years of age - do not use
Other information
- Store at room temeratur. Do not exceed 30ºC (86ºF)
- Lot No. & Exp. Date: see wrapper or box
Inactive ingredients
hydrogenated vegetable oil
CVS Bisacodyl Suppositories
CVSBisacodyl8ct.jpg
The product package shown above represents a sample of that currently in use. Additional packaging may also be available.
Bisacodyl Suppositories 8ct
Distributed by
CVS Pharmacy
One CVS Drive
Woonsocket, RI 02895
