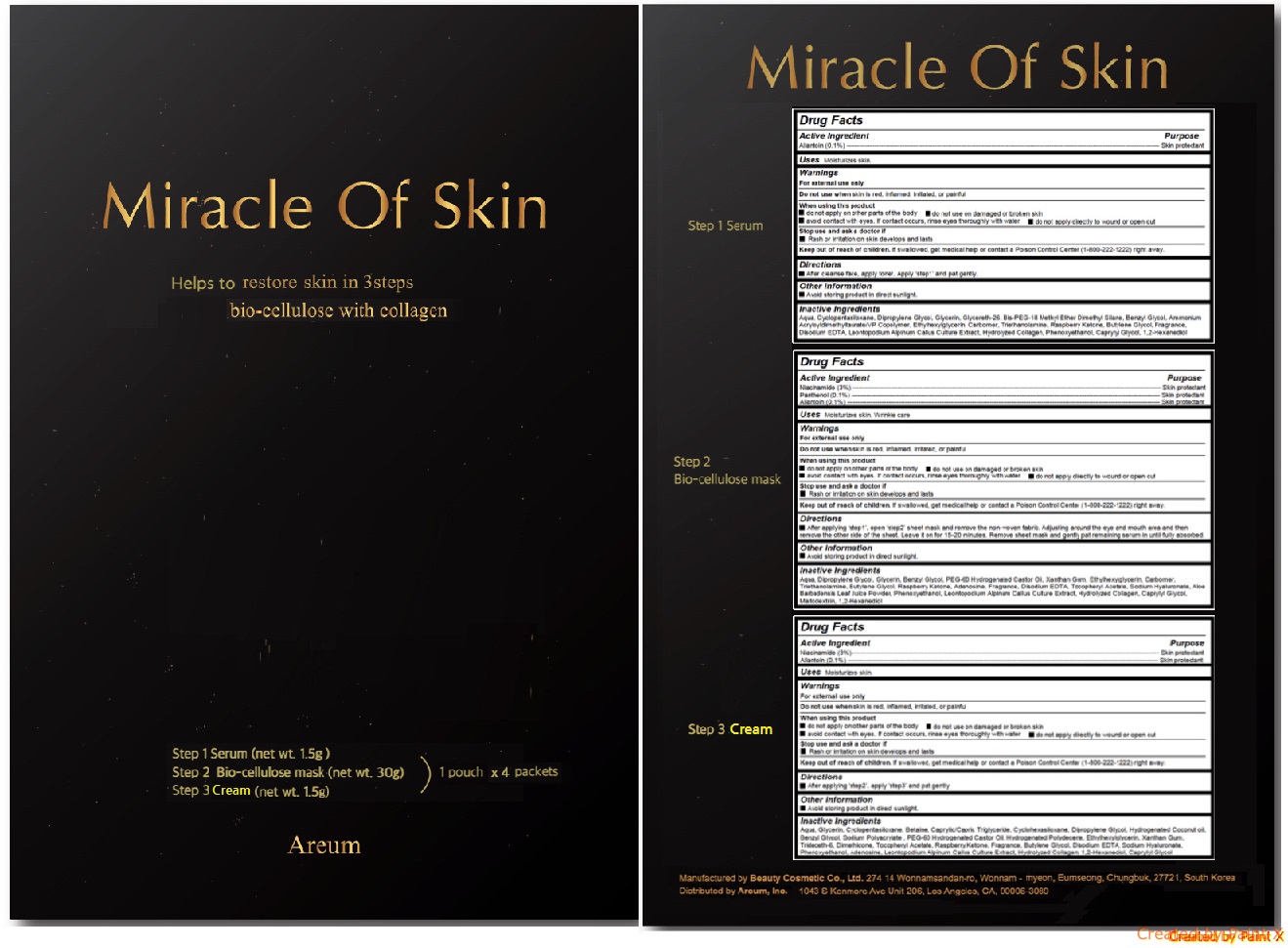
Active ingredients
Step 1 Serum - Allantoin (0.1%)
Step 2 Bio-cellulose mask - Niacinamide (3%), Panthenol (0.1%), Allantoin (0.1%)
Step 3 Cream - Niacinamide (3%), Allantoin (0.1%)
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Uses
Step 1 Serum - Moisturize skin.
Step 2 Bio-cellulose mask - Moisturize skin. Wrinkle care.
Step 3 Serum - Moisturize skin.
Warnings
For external use only
Do not use when skin is red, inflamed, irritated, or painful
When using this product
- do not apply on other parts of the body
- do not use on damaged or broken skin
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water
- do not apply directly to wound or open cut
Stop use and ask a doctor if
- Rash or irritation on skin develops and lasts
Directions
Step 1 Serum - After cleanse face, apply toner. Apply ‘step1’ and pat gently.
Step 2 Bio-cellulose mask - After applying ‘step1’, open ‘step2’ sheet mask and remove the non-woven fabric. Adjusting around the eye and mouth area and then remove the other side of the sheet. Leave it on for 15-20 minutes. Remove sheet mask and gently pat remaining serum in until fully absorbed.
Step 3 Serum - After applying ‘step2’, apply ‘step3’ and pat gently.
Step 1 Serum - Aqua, Cyclopentasiloxane, Dipropylene Glycol, Glycerin, Glycereth-26, Bis-PEG-18 Methyl Ether Dimethyl Silane, Benzyl Glycol, Ammonium Acryloyldimethyltaurate/VP Copolymer, Ethylhexylglycerin, Carbomer, Triethanolamine, Raspberry Ketone, Butylene Glycol, Fragrance, Disodium EDTA, Leontopodium Alpinum Callus Culture Extract, Hydrolyzed Collagen, Phenoxyethanol, Caprylyl Glycol, 1,2-Hexanediol
Step 2 Bio-cellulose mask - Aqua, Dipropylene Glycol, Glycerin, Benzyl Glycol, PEG-60 Hydrogenated Castor Oil, Xanthan Gum, Ethylhexylglycerin, Carbomer, Triethanolamine, Butylene Glycol, Raspberry Ketone, Adenosine, Fragrance, Disodium EDTA, Tocopheryl Acetate, Sodium Hyaluronate, Aloe Barbadensis Leaf Juice Powder, Phenoxyethanol, Leontopodium Alpinum Callus Culture Extract, Hydrolyzed Collagen, Caprylyl Glycol, Maltodextrin, 1,2-Hexanediol
Step 3 Serum - Aqua, Glycerin, Cyclopentasiloxane, Betaine, Caprylic/Capric Triglyceride, Cyclohexasiloxane, Dipropylene Glycol, Hydrogenated Coconut oil, Benzyl Glycol, Sodium Polyacrylate , PEG-60 Hydrogenated Castor Oil, Hydrogenated Polydecene, Ethylhexylglycerin, Xanthan Gum, Trideceth-6, Dimethicone, Tocopheryl Acetate, Raspberry Ketone, Fragrance, Butylene Glycol, Disodium EDTA, Sodium Hyaluronate, Phenoxyethanol, Adenosine, Leontopodium Alpinum Callus Culture Extract, Hydrolyzed Collagen, 1,2-Hexanediol, Caprylyl Glycol