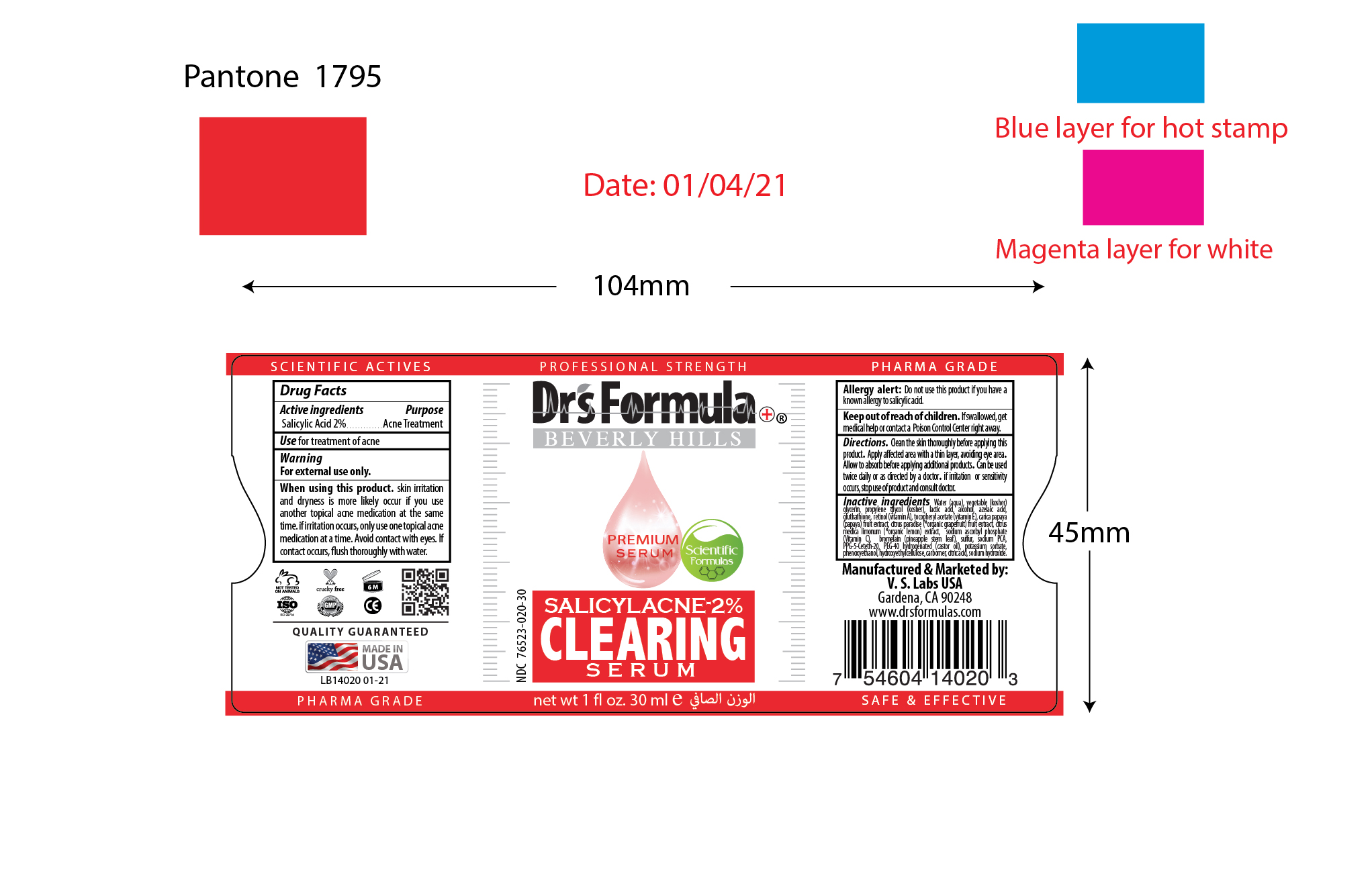
When using this product
Skin irritation and dryness is more likely occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time. Avoid contact with eyes. If contact occurs, flush thoroughly with water.
Stop use and ask doctor
If irritation or sensitivity occurs, stop use of product and consult doctor.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Clean the skin thoroughly before applying this product. Apply affected area with a thin layer, avoiding eye area. Allow to absorb before applying additional products. Can be used twice daily or as directed by a doctor.
Inactive ingredients
Water (aqua), vegetable (kosher) glycerin, propylene glycol (kosher), alcohol, retinol (vitamin A), tocopheryl acetate (vitamin E), azelaic acid, gluthathione, lactic acid, sodium ascorbyl phosphate (Vitamin C), carica papaya (papaya) fruit extract, citrus paradise (*organic grapefruit) fruit extract, citrus medica limonum (*organic lemon) extract, bromelain (*pineapple stem leaf), sulfur, sodium PCA, PPG-5-Ceteth-20, PEG-40 hydrogenated (castor oil), potassium sorbate, phenoxyethanol, hydroxyethylcellulose, carbomer, citric acid, sodium hydroxide