USE(S)
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
runny nose
itchy, watery eyes
sneezing
itching of the nose or throat
WARNINGS
Do not use
If you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have
Liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
Do not take more than directed. Taking more than directed may cause drowsiness.
Stop use and ask a doctor if
An allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding,
Ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
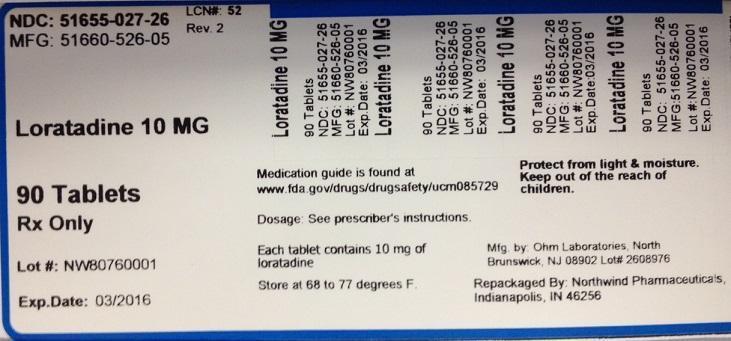
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 51655-027-26
MFG: 51660-526-05
Loratadine 10 MG
90 Tablets
Rx Only
Lot#
Exp. Date:
Medication guide is found at www.fda.gov/drugs/drugsafety/ucm085729
Dosage: See prescriber's instructions
Each tablet contains 10 mg of loratadine
Store at 68 to 77 degrees F.
Protect from light and moisture
Keep out of the reach of children.
Mfg. by Ohm Laboratories, North Brunswick, NJ 08902 Lot#
Repackaged by Northwind Pharmaceuticals, Indianapolis, IN 46256