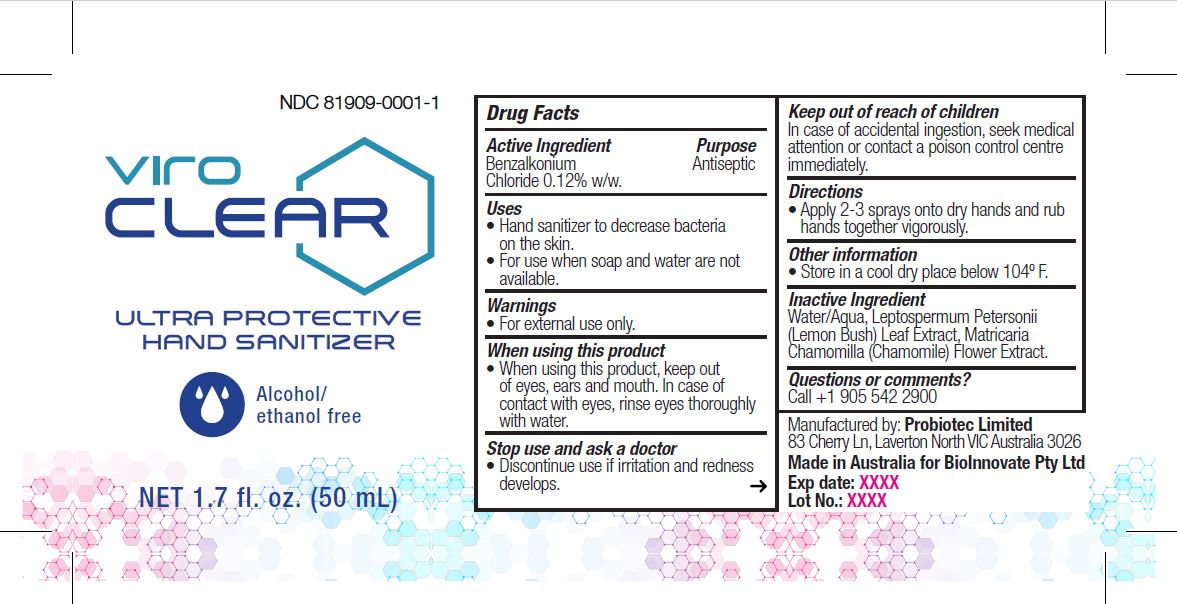
VIROCLEAR- benzalkonium chloride liquid
Bioinnovate Pty Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Benzalkonium Chloride 0.12% w/w.
Purpose
Antiseptic, Hand Sanitizer
Uses
- Hand sanitizer to decrease bacteria on the skin.
- For use when soap and water are not available.
Warnings
For external use only.
When using this product
When using this product, keep out of eyes, ears and mouth. In case ofcontact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor
Discontinue use if irritation and redness develops.
Keep out of reach of children
In case of accidental ingestion, seek medical attention or contact a poison control centre immediately.
Directions
Apply 2-3 sprays onto dry hands and rub hands together vigorously.
Other information
Store in a cool dry place below 104º F.
Inactive Ingredients
Water/Aqua, Leptospermum Petersonii (Lemon Bush) Leaf Extract, Matricaria Chamomilla (Chamomile) Flower Extract.
Questions or comments
Call +1 905 542 2900
Package Label - Principal Display Panel
50 mL; NDC 81909-0001-1