Keep Out of Reach of Children
In case of overdose, get medical help or contact a Poison Control Center right away.
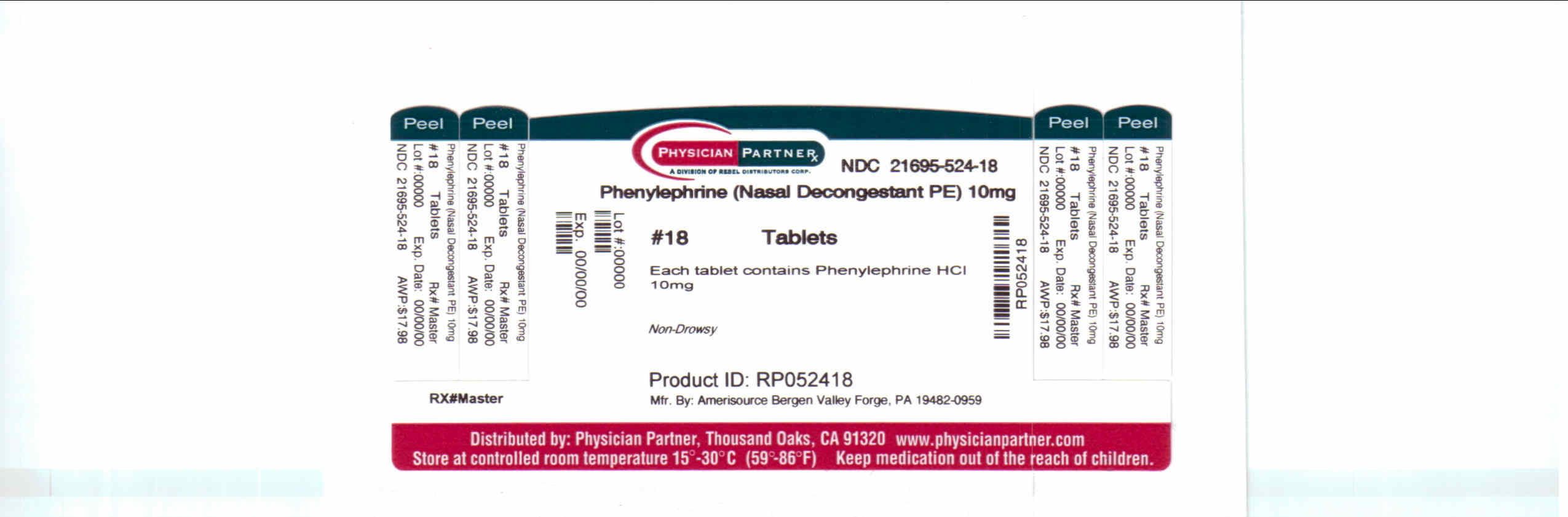
Uses
° temporarily relieves sinus congestion and pressure
° temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
Warnings
Do not use if you are now taking a prescription monoamine exidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have:
° heart disease ° high blood pressure ° diabetes ° trouble urinating due to an enlarge prostate gland ° thyroid disease
When using this product, do not use more than directed.
Stop use and ask a doctor if:
° you get nervous, dizzy or sleepless
° symptoms do not improve within 7 days or are accompanied by fever
If pregnant or breast-feeding, ask a health professional before use.
Directions
° take every 4 hours
° do not take more than 6 doses in 24 hours
° adults and children 12 years of age and over: 1 tablet
° children under 12 years of age: ask a doctor