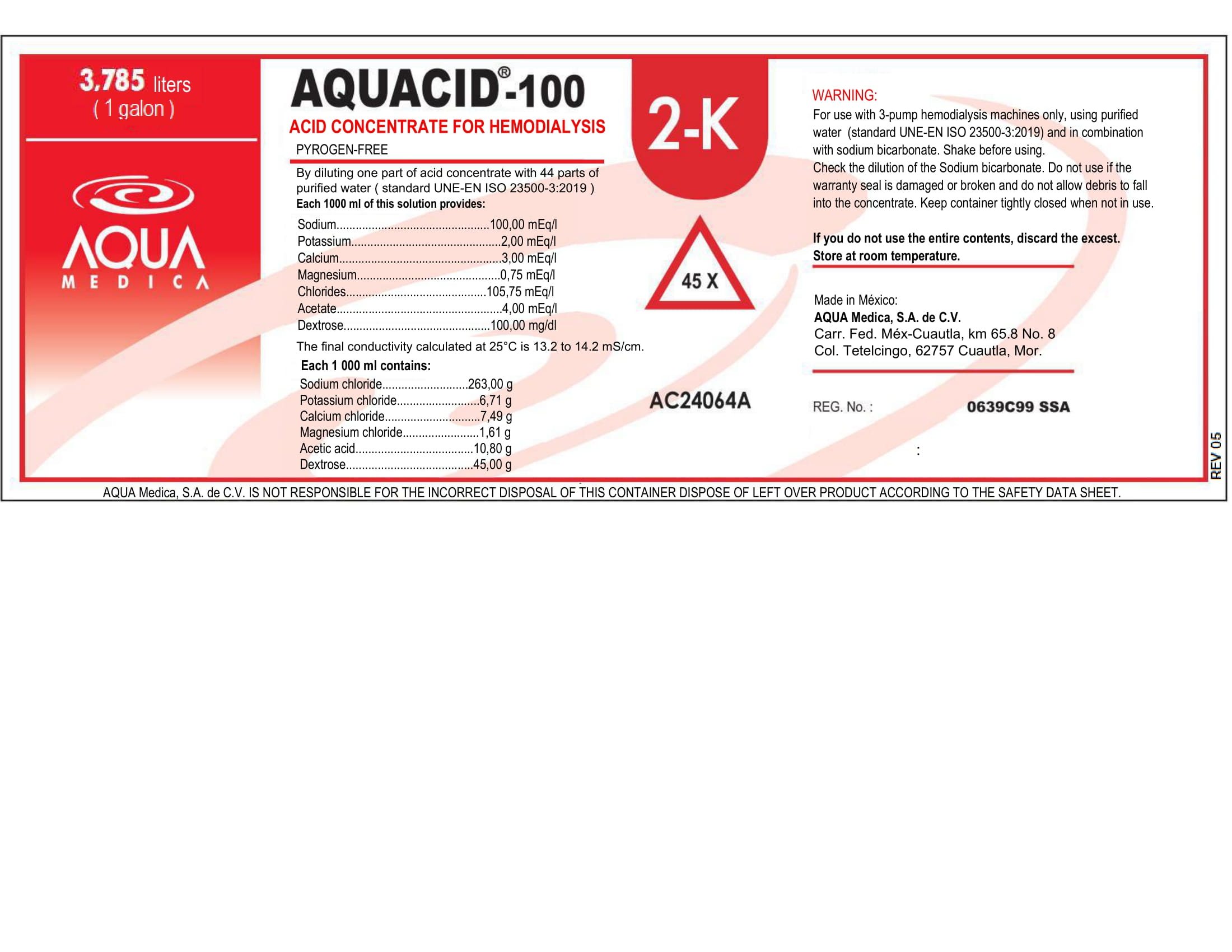
This is a Hemodialysis Acid Concentrate, Pyrogen-Free.
By diluiting one part of this acid concentrate with 44 parts of purified water (ISO Standard 13959:2014), each 1000 ml of this solucion provides:
Sodium: 100.00 mEq/l
Potassium: 2.00 mEq/l
Calcium: 3.00 mEq/l
Magnesium: 0.75 mEq/l
Chlorides: 105.75 mEq/l
Acetate: 4.00 mEq/l
Dextrose: 100.00 mEq/l
The final conductivity calculated at 25 C is 13.2 to 14.2 mS/cm.
Use
For use with 3-pump hemodialysis machines only, using purified water (Standard 13959:2014) and in combination with sodium bicarbonate.
Do not use
- If the warranty seal is damaged or broken and do not allow debris to fall into the concentrate.
Using purified water (Standard 13959:2014) and in combination with sodium bicarbonate.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
 3785 mL NDC: 81943-605-01
3785 mL NDC: 81943-605-01