DOCUSATE SODIUM- docusate sodium capsule Marlex Pharmaceuticals, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Docusate Sodium 100 mg
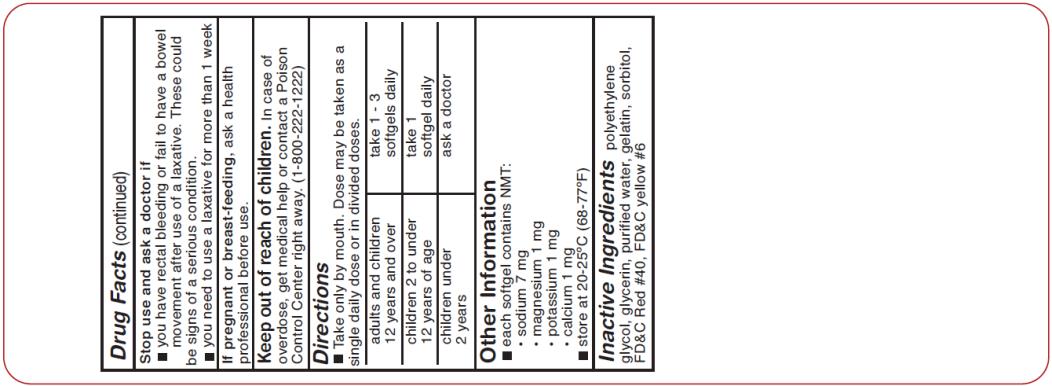
Drug Facts
Uses
- relieves occasional constipation (irregularity) generally produces bowel movement in 12 to 72 hours
Warnings
Ask a doctor before use if you have
- stomach pain
- Nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Ask a doctor or pharmacist before use if you are
taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
Directions
| adults and children 12 years and over | take 1 - 3 softgels once daily or in divded doses |
| children 2 to under 12 years of age | 1 softgel once daily |
| children under 2 years | ask a doctor |
Other informaiton
- each softgel contains: s
odium 7 mg
- store at room temperature 15º-25ºC (59º-77ºF) and avoid excessive heat
Inactive ingredients
FD&C red #40, FD&C yellow #6, gelatin, glycerin, polyethylene glycol 400 NF, purified water, sorbitol
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SEAL UNDER CAP IS BROKEN OR MISSING.
*Marlex Pharmaceuticals, Inc., is not affiliated with the owner of the trademark Colace®
Distributed by:
Marlex Pharmaceuticals, Inc.
New Castle, DE 19720
Made in Romania