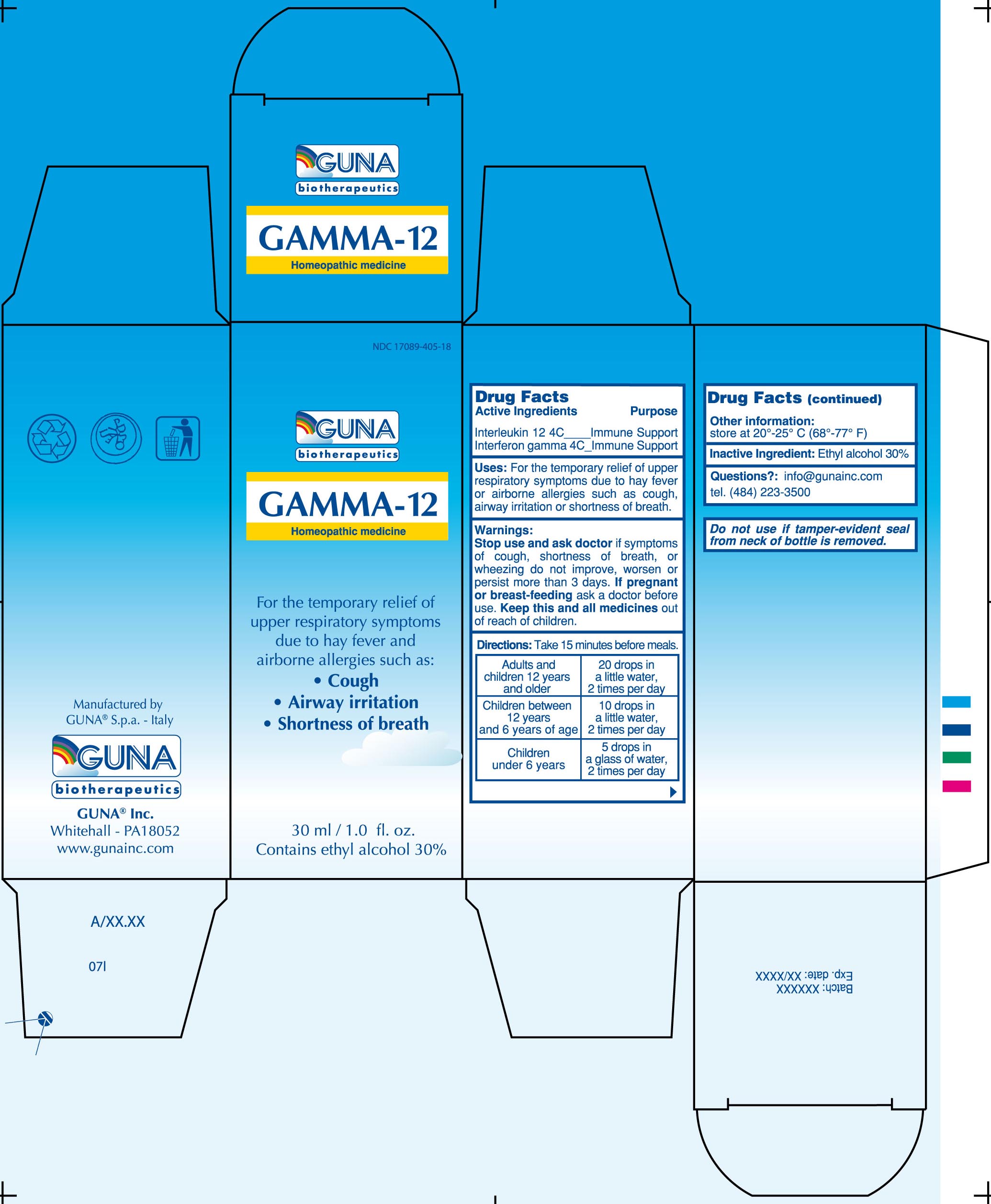
GAMMA -12- interleukin-12 human recombinant - interferon gamma-1b - solution/ drops
Guna spa
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENTS/PURPOSE
INTERFERON GAMMA 4C IMMUNE SUPPORT
INTERLEUKIN-12 4C IMMUNE SUPPORT
USES
For the temporary relief of upper respiratory symptoms due to hay fever or airborne allergies such as: -Cough –Airway irritation –Shortness of breath
WARNINGS
Stop use and ask doctor if symptoms of cough, shortness of breath, or wheezing do not improve, worsen or persist more than 3 days
PREGNANCY
If pregnant or breast-feeding ask a doctor before use
WARNINGS
Keep this and all medicines out of reach of children
DIRECTIONS
Take 15 minutes before meals
Adults and children 12 years and older
20 drops in a little water, 2 times per day
Children between 12 years and 6 years of age
10 drops in a little water, 2 times per day
Children under 6 years
5 drops in a glass of water, 2 times per day
QUESTIONS
Questions?: info@gunainc.com
tel. (484) 223-3500
inactive ingredient: Ethyl alcohol 30%.
take 15 minutes before meals
PRINCIPAL DISPLAY PANEL