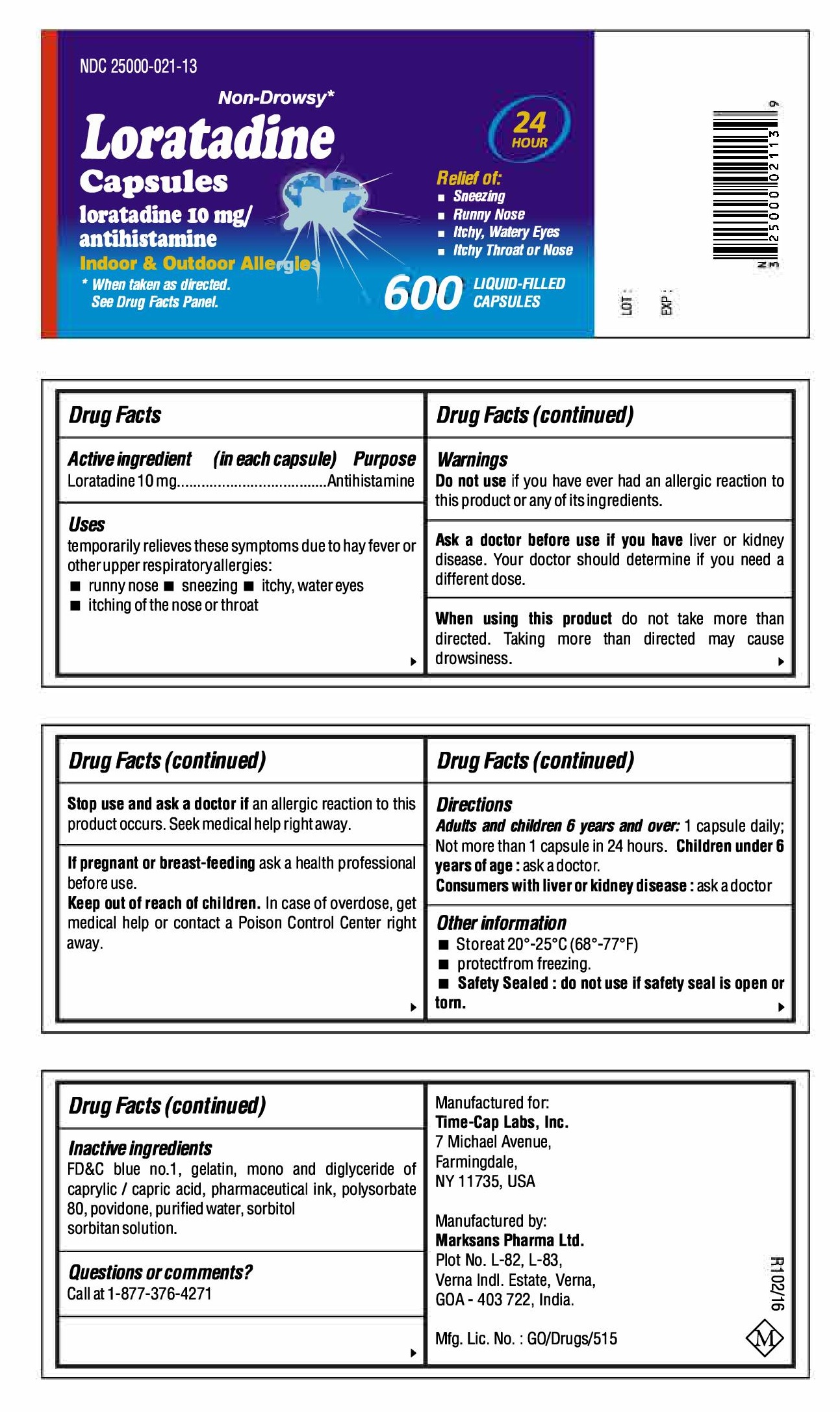
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
• runny nose
• itchy, watery eyes
• sneezing
• itching of the nose or throat
Ask a doctor before use if you have
you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 6 years and over: 1 capsule daily; Not more than 1 capsule in 24 hours.
Children under 6 years of age: ask a doctor.
Consumers with liver or kidney disease: ask a doctor.
Inactive ingredients
FD&C blue no. 1, gelatin, mono and diglyceride of caprylic/capric acid, pharmaceutical ink, polysorbate 80, povidone, purified water, sorbitol sorbitan solution.
Questions or comments?
Call at 1-877-376-4271
Other information
- Store at 20°-25°C (68°-77°F)
- protect from freezing.
- Safety sealed: do not use if the individual blister unit imprinted with Loratadine Capsules is open or torn.
Manufactured by
Marksans Pharma Ltd.
Plot No. L-82, L-83,
Verna Indl. Estate,
Verna, GOA 403722, India.