CAFFEINE- caffeine tablet
Walgreen Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
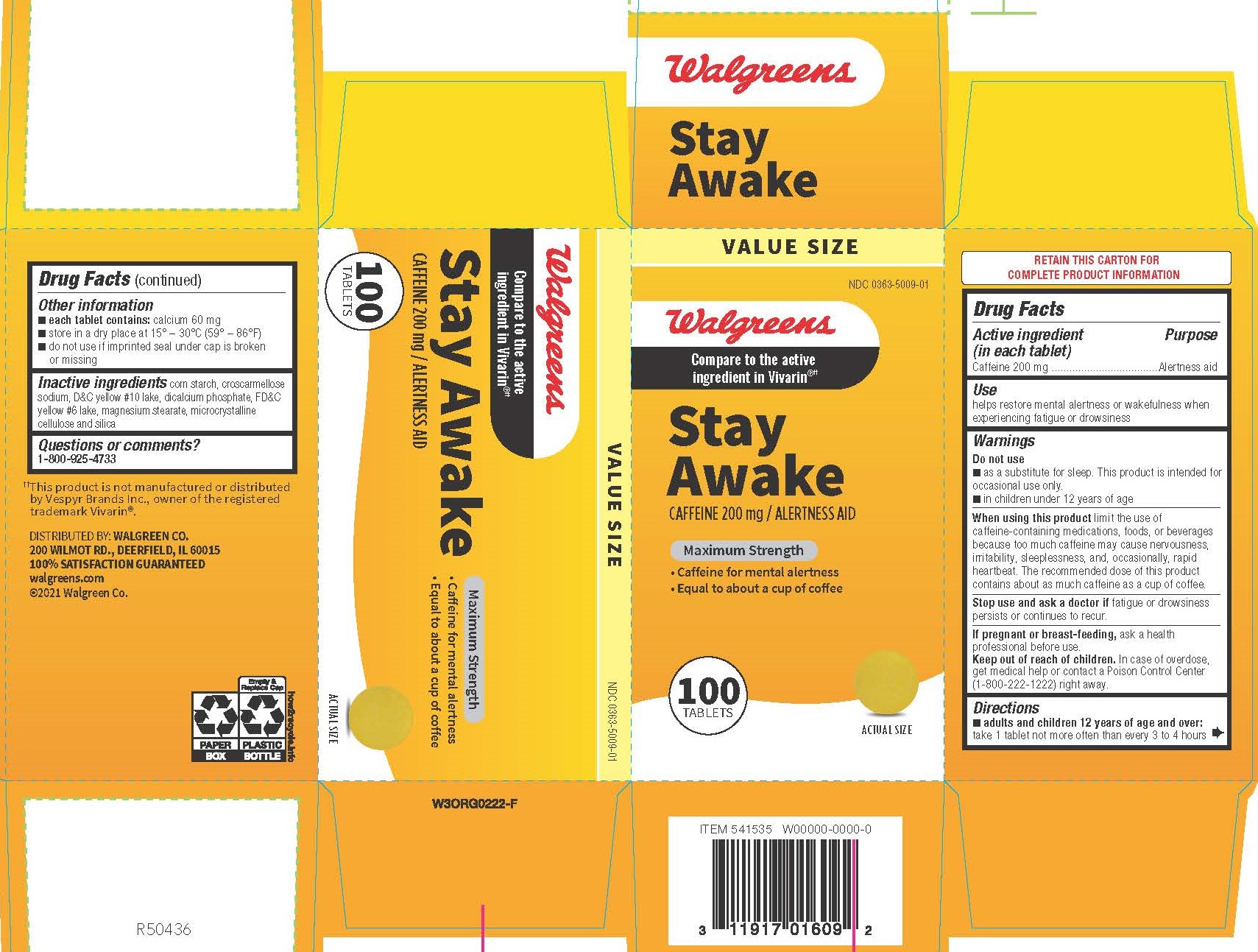
Drug Facts
Active ingredient (in each tablet)
Caffeine 200 mg
Use
helps restore mental alertness or wakefulness when experiencing fatigue or drowsiness
Do not use
■ as a substitute for sleep. This product is intended for occasional use only.
■ in children under 12 years of age
When using this product limit the use of caffeine-containing medications, foods, or beverages because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heartbeat. The recommended dose of this product contains about as much caffeine as a cup of coffee.
Stop use and ask a doctor if fatigue or drowsiness persists or continues to recur.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
■ adults and children 12 years of age and over: take 1 tablet not more often than every 3 to 4 hours
Other Information
■ each tablet contains: calcium 60 mg
■ store in a dry place at 15° – 30°C (59° – 86°F)
■ do not use if imprinted seal under cap is broken or missing
corn starch, croscarmellose sodium, D&C yellow #10 lake, dicalcium phosphate, FD&C yellow #6 lake, magnesium stearate, microcrystalline cellulose and silica
Questions or comments?
1-800-925-4733
DISTRIBUTED BY:
WALGREEN CO.
200 WILMOT ROAD
DEERFIELD, IL 60015
Walgreens
Compare to Vivarin® active ingredient††
Stay Awake
CAFFEINE 200 mg /
ALERTNESS AID
Maximum Strength
• Caffeine for mental alertness
• Equal to about a cup of coffee
100 Tablets