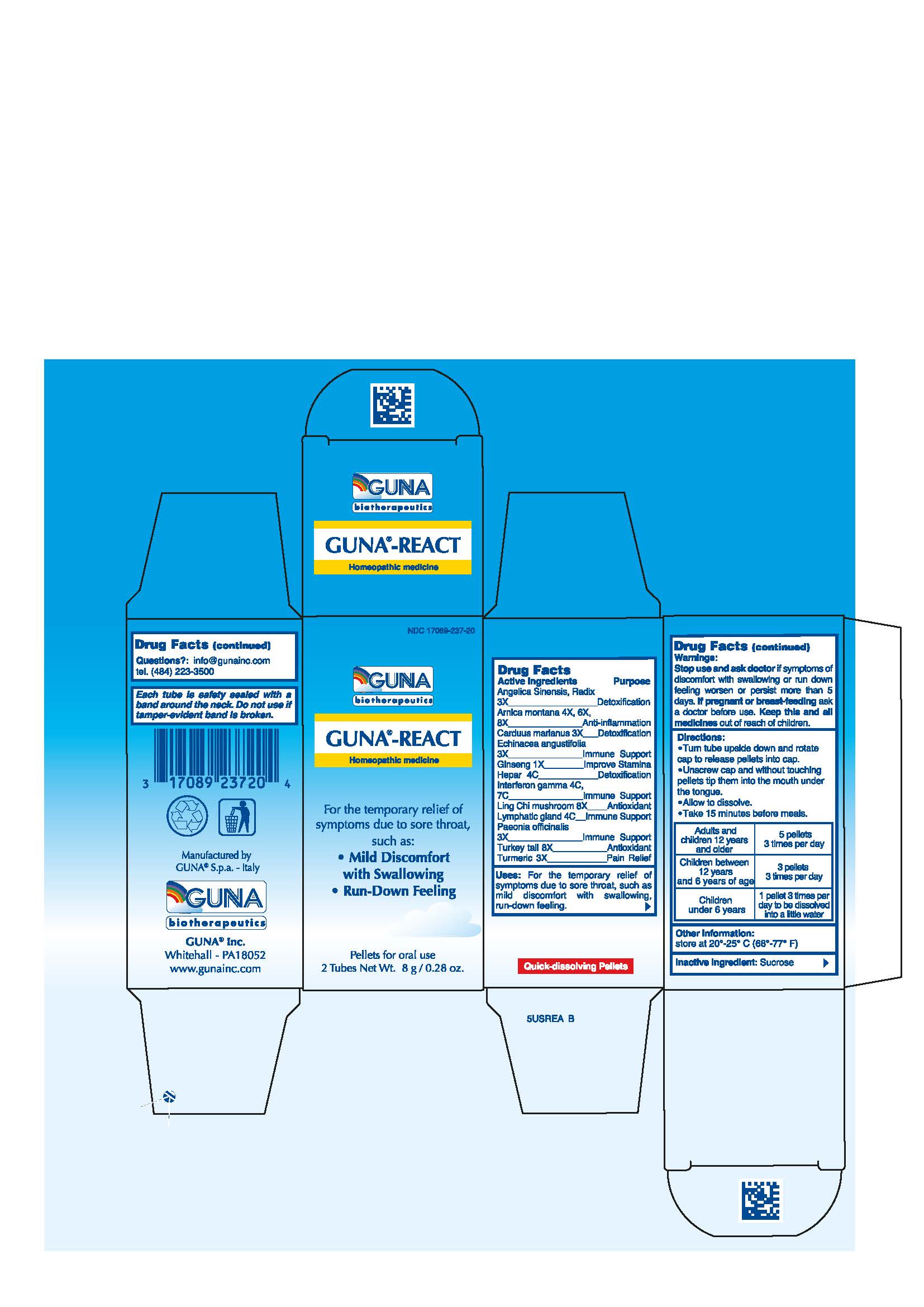
ACTIVE INGREDIENTS/PURPOSE
ANGELICA SINENSIS, RADIX 3X DETOXIFICATION
ARNICA MONTANA 4X, 6X, 8X ANTI-INFLAMMATION
CARDUUS MARIANUS 3X DETOXIFICATION
ECHINACEA ANGUSTIFOLIA 3X IMMUNE SUPPORT
GINSENG 1X IMPROVE STAMINA
HEPAR 4C DETOXIFICATION
INTERFERON GAMMA 4C 7C IMMUNE SUPPORT
LING CHI MUSHROOM 8X ANTIOXIDANT
LYMPHATIC GLAND 4C IMMUNE SUPPORT
PAEONIA OFFICINALIS 3X IMMUNE SUPPORT
TURKEY TAIL 8X ANTIOXIDANT
TURMERIC 3X PAIN RELIEF
USES
For the temporary relief of symptoms due to sore throat, such as: •Mild Discomfort with Swallowing •Run-Down Feeling
WARNINGS
Stop use and ask doctor if symptoms of discomfort with swallowing or run down feeling worsen or persist more than 5 days
DIRECTIONS
Take 15 minutes before meals
Adults and children 12 years and older 5 pellets 3 times per day
Children between 12 years and 6 years of age 3 pellets 3 times per day
Children under 6 years 1 pellet 3 times per day to be dissolved into a little water