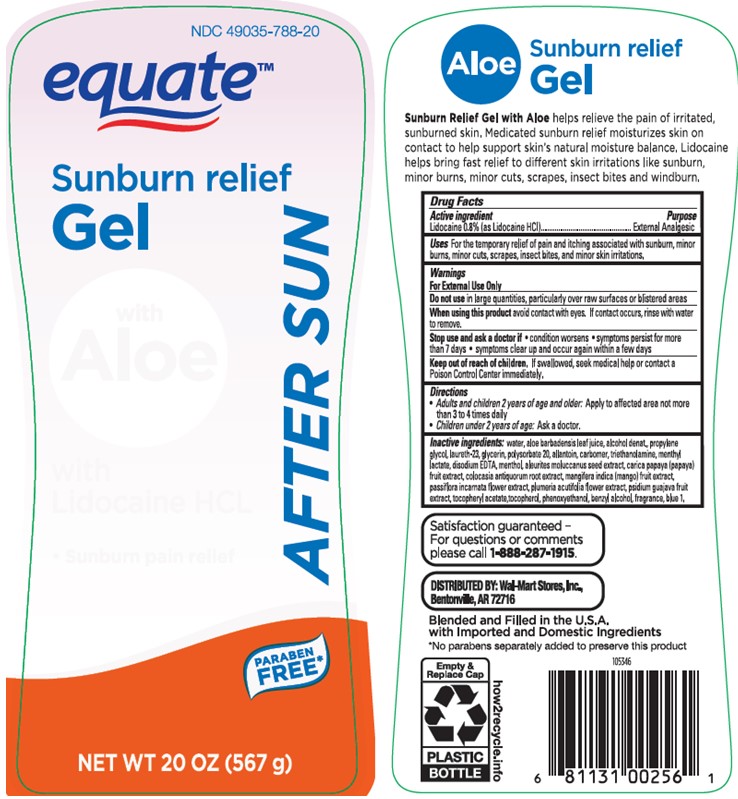
Uses
For the temporary relief of pain and itching associated with sunburn, minor burns, minor cuts, scrapes, insect bites, and minor skin irritations
Stop use and ask a doctor if
• condition worsens • symptoms persist for more than 7 days • symptoms clear up and occur again within a few days
Keep out of reach of children
If swallowed, seek medical help or contact a Poison Control Center immediately.
Directions
• Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily
• Children under 2 years of age: Ask a doctor.
Inactive ingredients
water, aloe barbadensis leaf juice, alcohol denat., propylene glycol, laureth-23, glycerin, polysorbate 20, allantoin, carbomer, triethanolamine, menthyl lactate, disodium EDTA, menthol, aleurites moluccanus seed extract, carica papaya (papaya) fruit extract, colocasia antiquorum root extract, mangifera indica (mango) fruit extract, passiflora incarnata flower extract, plumeria acutifolia flower extract, psidium guajava fruit extract, tocopheryl acetate, tocopherol, phenoxyethanol, benzyl alcohol, fragrance, blue 1.