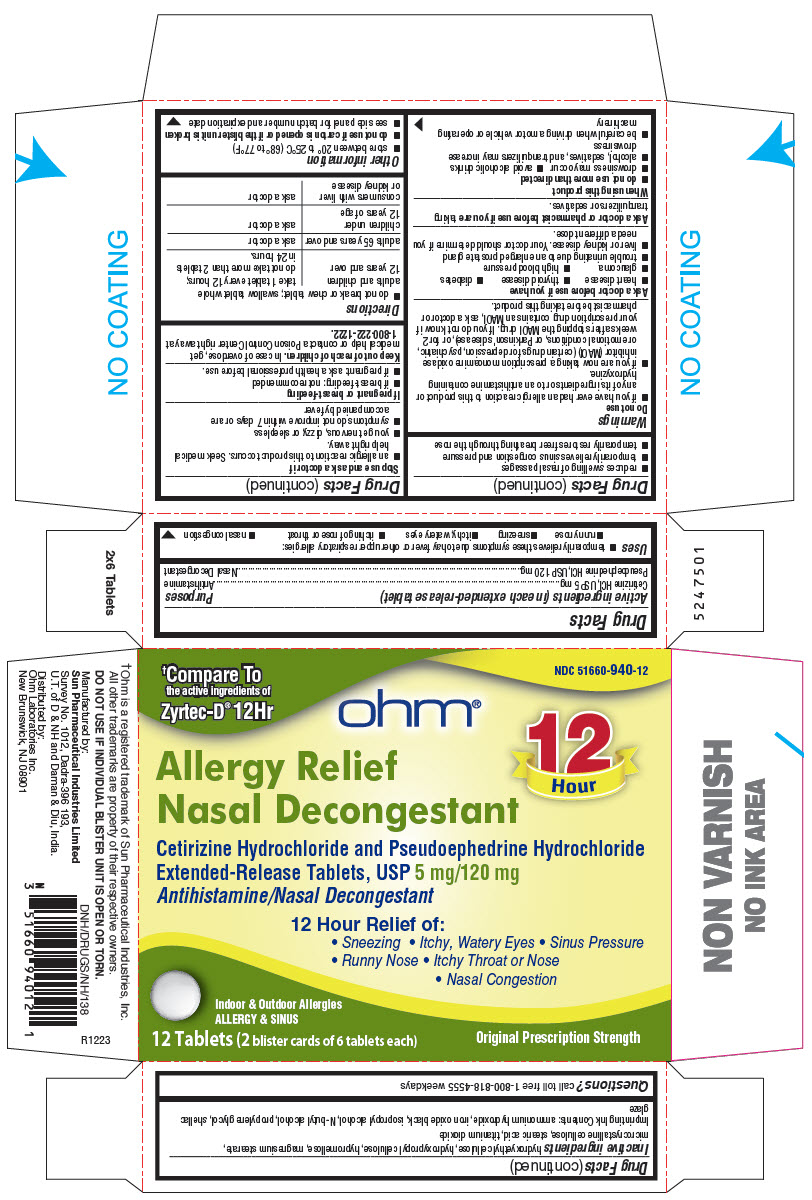
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of nose or throat
- nasal congestion
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily restores freer breathing through the nose
Warnings
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- thyroid disease
- diabetes
- glaucoma
- high blood pressure
- trouble urinating due to an enlarged prostate gland
- liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not use more than directed
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- an allergic reaction to this product occurs. Seek medical help right away.
- you get nervous, dizzy, or sleepless
- symptoms do not improve within 7 days or are accompanied by fever
Directions
- do not break or chew tablet; swallow tablet whole
| adults and children 12 years and over | take 1 tablet every 12 hours; do not take more than 2 tablets in 24 hours. |
| adults 65 years and over | ask a doctor |
| children under 12 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
Other information
- store between 20° to 25°C (68° to 77°F)
- do not use if carton is opened or if the blister unit is broken
- see side panel for batch number and expiration date
Inactive ingredients
hydroxyethyl cellulose, hydroxypropyl cellulose, hypromellose, magnesium stearate, microcrystalline cellulose, stearic acid, titanium dioxide
Manufactured by:
Sun Pharmaceutical Industries Limited
Survey No. 1012, Dadra-396 193,
U.T. of D & NH and Daman & Diu, India.
Distributed by:
Ohm Laboratories Inc.
New Brunswick, NJ 08901
PRINCIPAL DISPLAY PANEL - 12 Tablet Blister Card Carton
†Compare To
the active ingredients of
Zyrtec-D®12Hr
NDC 51660-940-12
ohm®
12
Hour
Allergy Relief
Nasal Decongestant
Cetirizine Hydrochloride and Pseudoephedrine Hydrochloride
Extended-Release Tablets, USP 5 mg/120 mg
Antihistamine/Nasal Decongestant
12 Hour Relief of:
• Sneezing • Itchy, Watery Eyes • Sinus Pressure
• Runny Nose • Itchy Throat or Nose
• Nasal Congestion
Indoor & Outdoor Allergies
ALLERGY & SINUS
12 Tablets (2 blister cards of 6 tablets each)
Original Prescription Strength