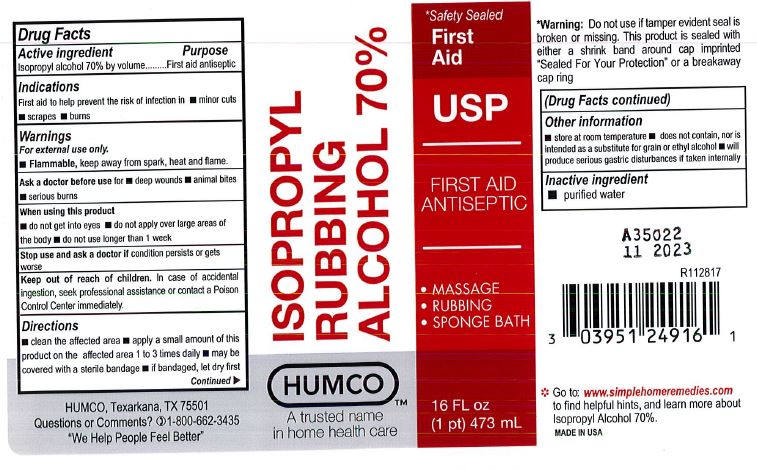
Active Ingredient
Isopropyl Alcohol 70% by volume
Purpose
First aid antiseptic
Use
First aid to help prevent the risk of infection in.
Warnings
For external use only.
- Flammable, keep way from spark, heat and flame.
Ask a doctor before use for
- deep wounds
- animal bites
- serious burns
When using this product
- do not get into eyes
- do not apply over large areas of the body
- do not use longer than 1 week
Stop use and ask a doctor if
condition persists or gets worse
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions
- clean the affected area.
- apply a smal amount of this product on the affected area 1 to 3 times daily.
- may be covered with sterile bandage.
- if bandaged, let it dry first.
Other Information
- store at room temperature
- does not contain, nor is intended as a substitute for grain or ethyl alcohol.
- will produce serious gastric distrurbances if taken internally.
Inactive Ingredient
purified water
Principal Display Panel
Humco Holding Group, Inc.
