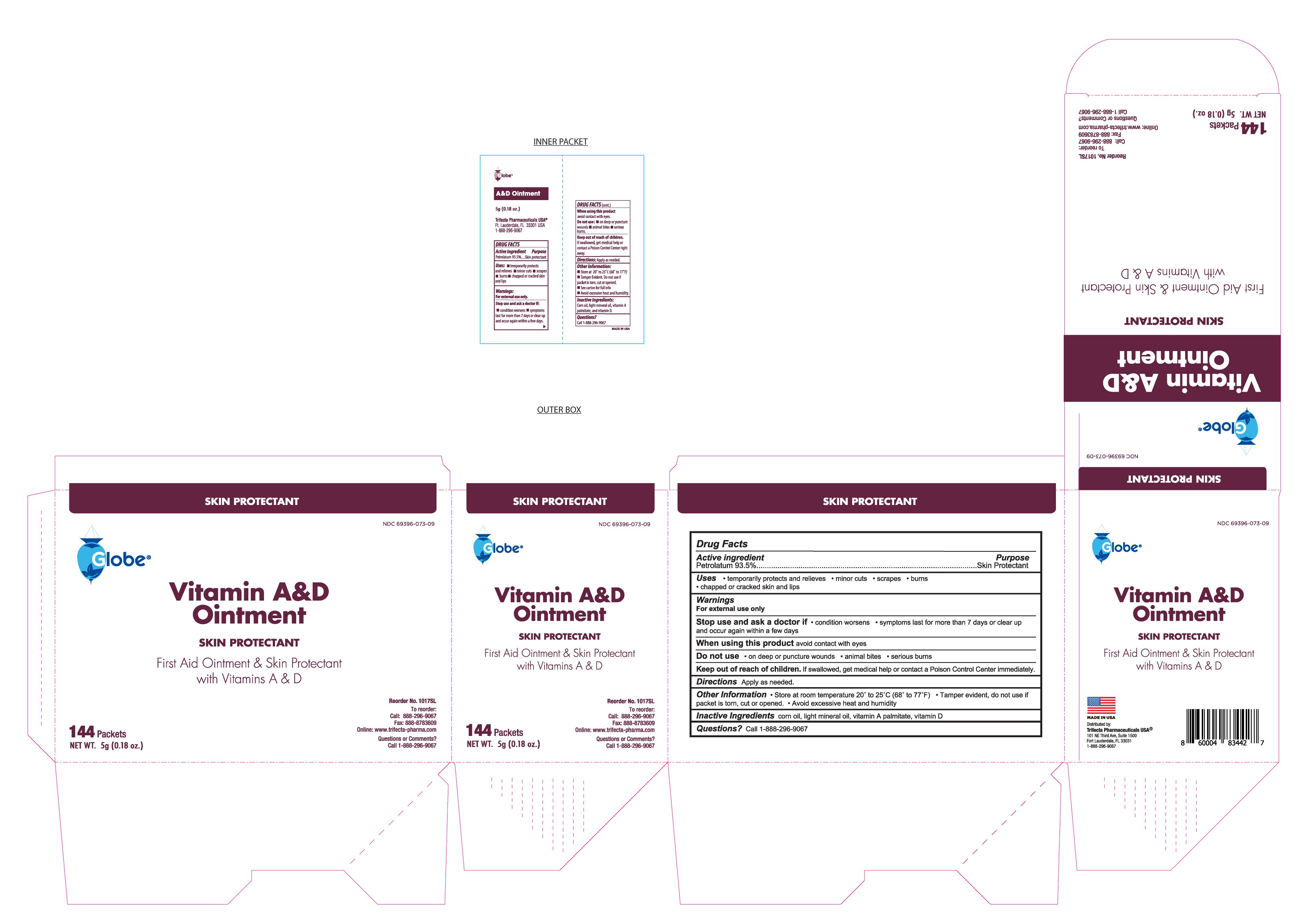
VITAMIN A AND D- petrolatum ointment
Trifecta Pharmaceuticals USA LLC
----------
Globe Vitamin A & D Ointment
Stop use and ask a doctor
Condition worsens
Symptoms last more than 7 days or clear up and occur again with a few days
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center immediately
Distributed By
Trifecta Pharmaceuticals USA
101 NE Third Avenue, Suite 1500
Ft. Lauderdale, FL. 33301
www.trifecta-pharma.com
1-888-296-9067
| VITAMIN A AND D
petrolatum ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Trifecta Pharmaceuticals USA LLC (079424163) |
Revised: 12/2023
Document Id: 0bc88177-4508-18a8-e063-6394a90a9cb0
Set id: c20fce25-8123-fe5d-e053-2a95a90a53a0
Version: 2
Effective Time: 20231205
Trifecta Pharmaceuticals USA LLC