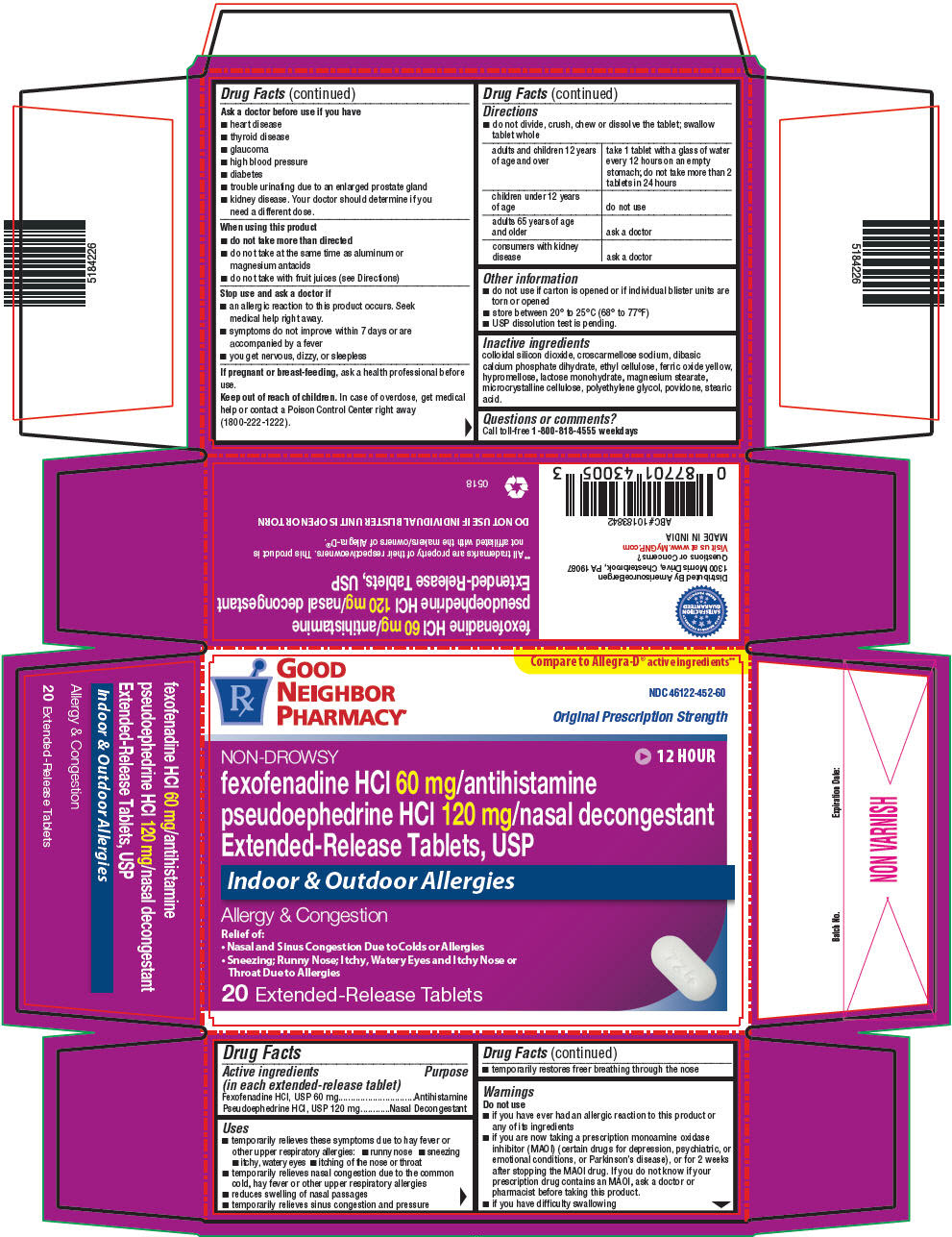
Active ingredient(s)
(in each extended-release tablet)
Fexofenadine HCl, USP 60 mg
Pseudoephedrine HCl, USP 120 mg
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily restores freer breathing through the nose
Do not use
-
if you have ever had an allergic reaction to this product or any of its ingredients -
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product. -
if you have difficulty swallowing
Ask a doctor before use if you have
- heart disease
- thyroid disease
- glaucoma
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate gland
- kidney disease. Your doctor should determine if you need a different dose.
When using this product
-
do not take more than directed -
do not take at the same time as aluminum or magnesium antacids -
do not take with fruit juices (see Directions)
Stop use and ask doctor if
-
an allergic reaction to this product occurs. Seek medical help right away. -
symptoms do not improve within 7 days or are accompanied by a fever -
you get nervous, dizzy, or sleepless
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
-
do not divide, crush, chew or dissolve the tablet; swallow tablet whole
| adults and children 12 years of age and over | take 1 tablet with a glass of water every 12 hours on an empty stomach; do not take more than 2 tablets in 24 hours |
| children under 12 years of age | do not use |
| adults 65 years of age and older | ask a doctor |
| consumers with kidney disease | ask a doctor |
Other information
- safety sealed; do not use if inner safety seal is open or torn
- store between 20° to 25°C (68° to 77°F)
- USP dissolution test is pending.
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, dibasic calcium phosphate dihydrate, ethyl cellulose, ferric oxide yellow, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, stearic acid.
PRINCIPAL DISPLAY PANEL - 60 mg/120 mg Tablet Blister Pack Carton
GOOD
NEIGHBOR
PHARMACY®
Compare to Allegra-D® active ingredients**
NDC 46122-452-60
Original Prescription Strength
NON-DROWSY
12 HOUR
fexofenadine HCl 60 mg/antihistamine
pseudoephedrine HCl 120 mg/nasal decongestant
Extended-Release Tablets, USP
Indoor & Outdoor Allergies
Allergy & Congestion
Relief of:
- Nasal and Sinus Congestion Due to Colds or Allergies
-
Sneezing; Runny Nose; Itchy, Watery Eyes and Itchy Nose or
Throat Due to Allergies
20 Extended-Release Tablets