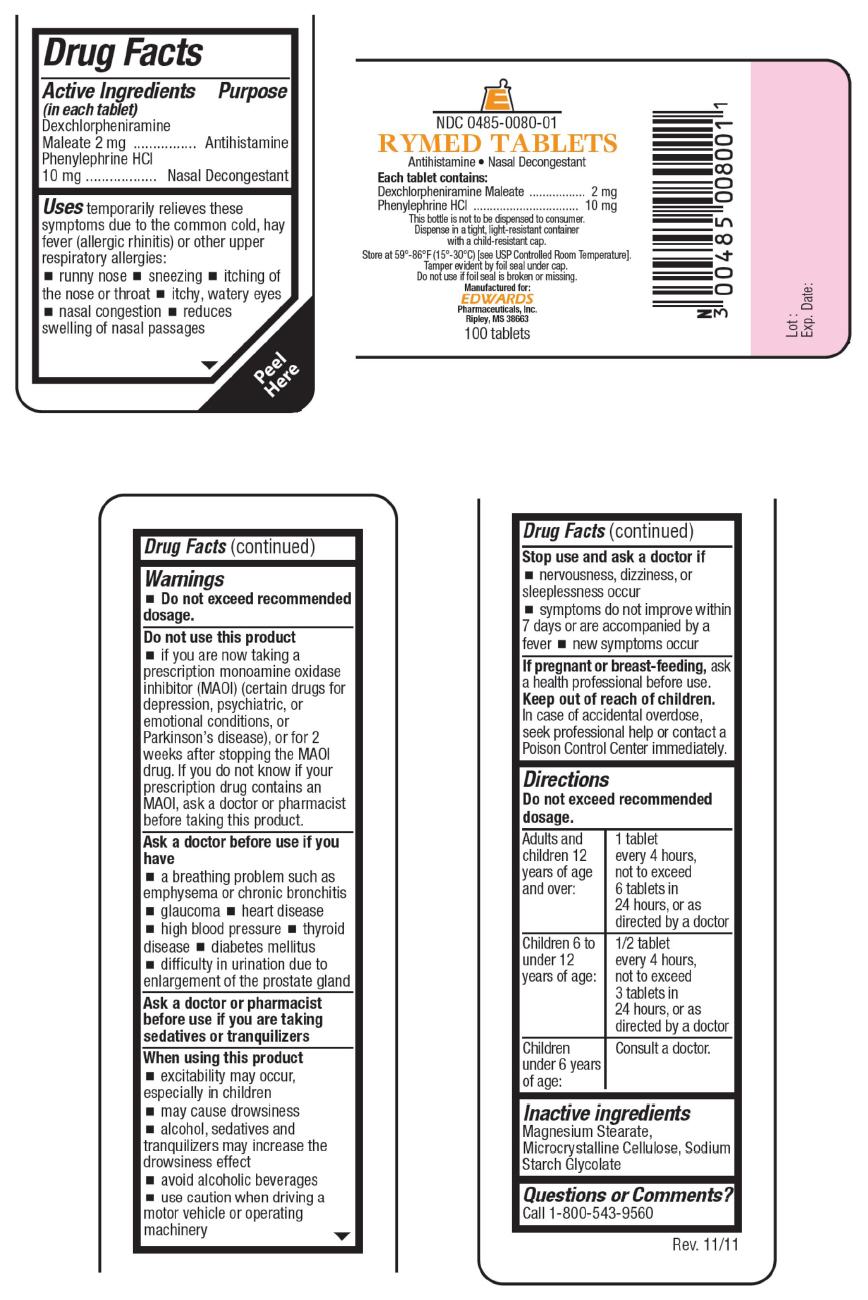
Uses
temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
- nasal congestion
- reduces swelling of nasal passages
Warnings
- Do not exceed recommended dosage.
Do not use this product
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- heart disease
- high blood pressure
- thyroid disease
- diabetes mellitus
- difficulty in urination due to enlargement of the prostate gland
When using this product
- excitability may occur, especially in children
- may cause drowsiness
- alcohol, sedatives and tranquilizers may increase the drowsiness effect
- avoid alcoholic beverages
- use caution when driving a motor vehicle or operating machinery
Directions
Do not exceed recommended dosage.
| Adults and children 12 years of age and over: | 1 tablet every 4 hours, not to exceed 6 tablets in 24 hours, or as directed by a doctor |
| Children 6 to under 12 years of age: | 1/2 tablet every 4 hours, not to exceed 3 tablets in 24 hours, or as directed by a doctor |
| Children under 6 years of age: | Consult a doctor. |
PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
E
NDC 0485-0080-01
RYMED TABLETS
Antihistamine • Nasal Decongestant
Each tablet contains:
Dexchlorpheniramine Maleate 2 mg
Phenylephrine HCl 10 mg
This bottle is not to be dispensed to consumer.
Dispense in a tight, light-resistant container
with a child-resistant cap.
Store at 59°-86° (15°-30°C) [see USP Controlled Room Temperature].
Tamper evident by foil seal under cap.
Do not use if foil seal is broken or missing.
Manufactured for:
EDWARDS
Pharmaceuticals, Inc.
Ripley, MS 38663
100 tablets