DESCRIPTION
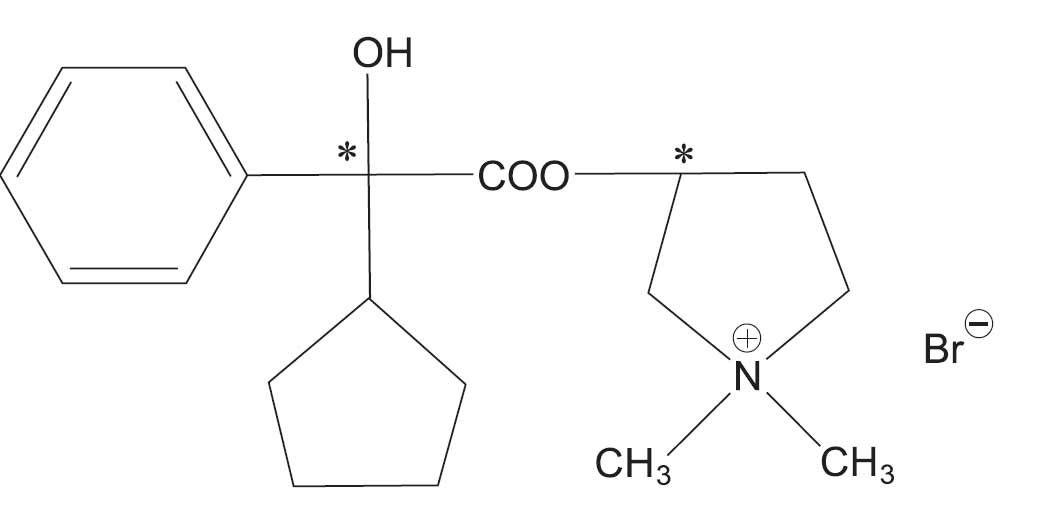
Glycopyrrolate tablets contain the synthetic anticholinergic glycopyrrolate. Glycopyrrolate is a quaternary ammonium compound with the following chemical name: 3-[(cyclopentylhydroxyphenylacetyl)oxy]-1,1-
dimethylpyrrolidinium bromide. Its empirical formula is C19H28BrNO3, its molecular weight is 398.33, and its structural formula is:
Each 1 mg tablet contains: Glycopyrrolate, USP ................1 mg
Each 2 mg tablet contains: Glycopyrrolate, USP ................2 mg
Inactive Ingredients: Dibasic Calcium Phosphate, Lactose, Magnesium Stearate, Povidone, Sodium Starch Glycolate.

