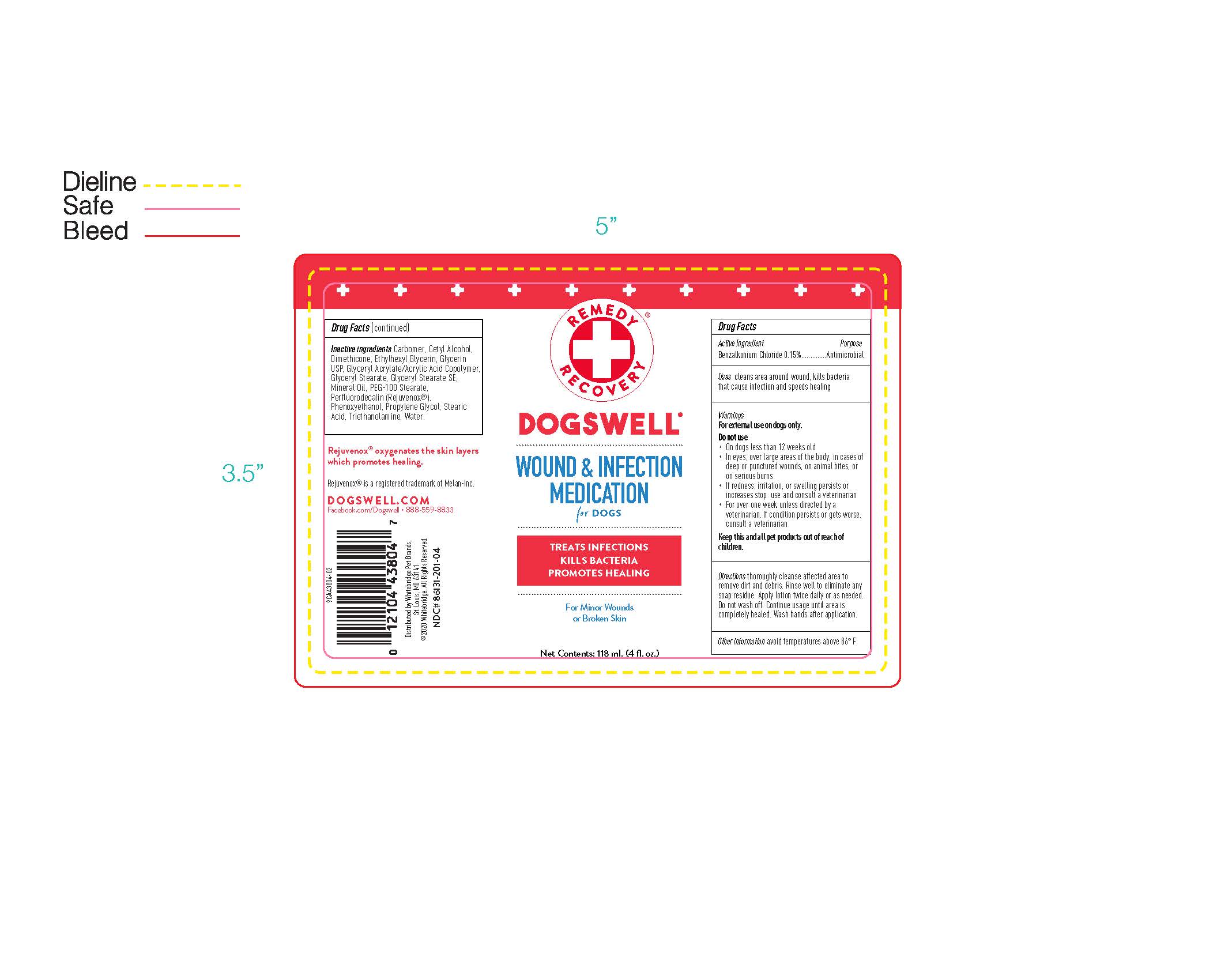
Warnings
For external use on dogs only.
Do not use
- On dogs less than 12 weeks old
- In eyes, over large areas of the body, in cases of deep or punctured wounds, on animal bites, or on serious burns
- If redness, irritation, or swelling persists or increases stop use and consult a veterinarian
- For over one week unless directed by a veterinarian. If condition persists or gets worse, consult a veterinarian
Directions
thoroughly cleanse affected area to remove dirt and debris. Rinse well to eliminate any soap residue. Apply lotion twice daily or as needed. Do not wash off. Continue usage until area is completely healed. Wash hands after application.
Drug Facts (contiuned)
Inactive ingredients Carbomer, Cetyl Alcohol, Dimethicone, Ethylhexyl Glycerin, Glycerin USP, Glyceryl Acrylate/Acrylic Acid Copolymer, Glyceryl Stearate, Glyceryl Stearate SE, Mineral Oil, PEG-100 Stearate, Perflurodecalin (Rejuvenox), Phenoxyethanol, Propylene Glycol, Stearic Acid, Triethanolamine, Water.
Rejuvenox oxygenates the skin layers which promotes healing.
Rejuvenox is a registered trademark of Melan-Inc.
DOGSWELL.COM
Facebook.com/Dogswell 888-559-8833