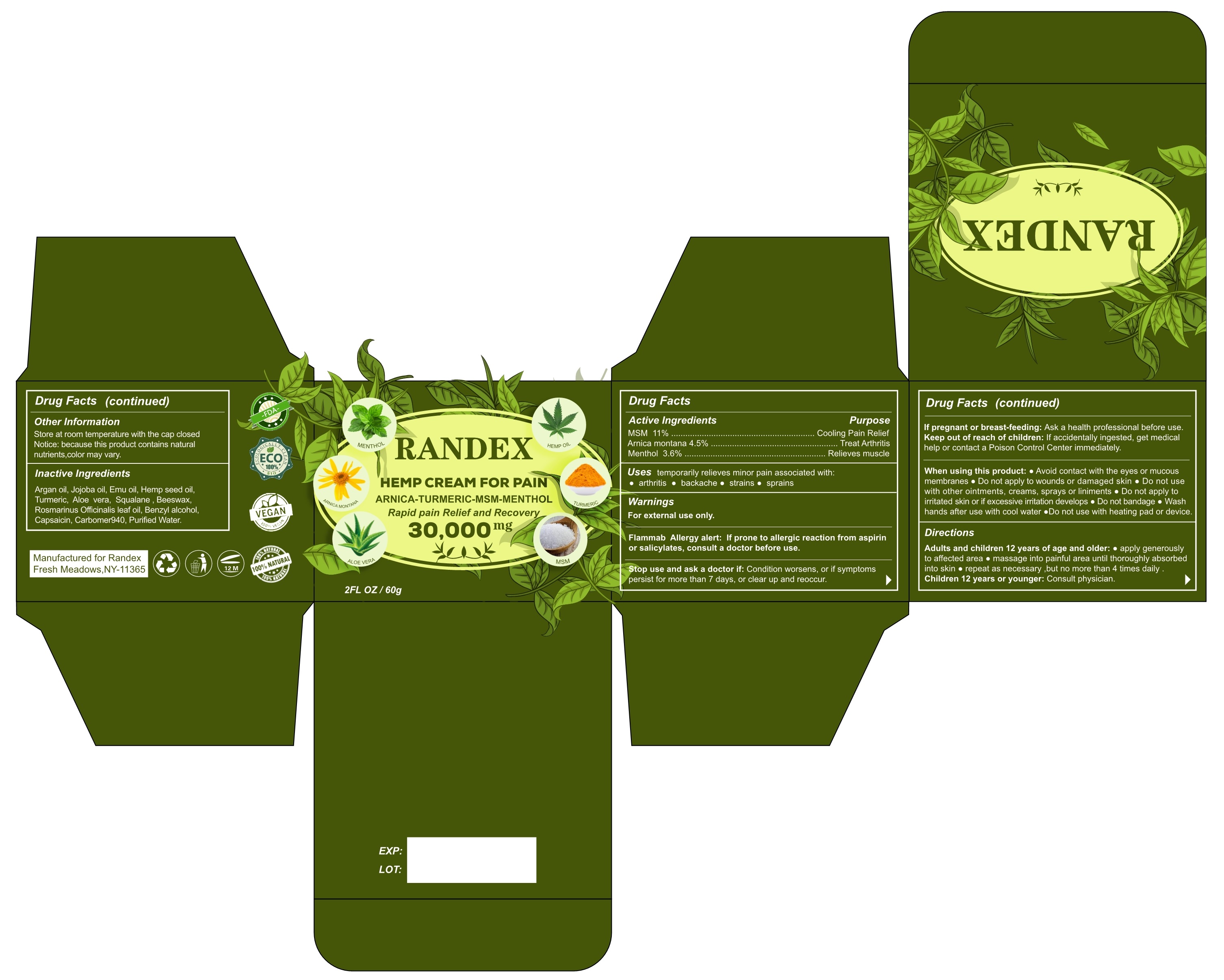
Active Ingredient(s)
MSM 11% ............................................................. Cooling Pain Relief
Arnica montana 4.5% ...................................................... Treat Arthritis
Menthol 3.6% ............................................................ Relieves muscle
Purpose
Active Ingredients Purpose
MSM 11% ............................................................. Cooling Pain Relief
Arnica montana 4.5% ...................................................... Treat Arthritis
Menthol 3.6% ............................................................ Relieves muscle
Use
Flammab Allergy alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
Warnings
For external use only.
Flammab Allergy alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
Stop use and ask a doctor if: Condition worsens, or if symptoms persist for more than 7 days, or clear up and reoccur.
If pregnant or breast-feeding: Ask a health professional before use.
Keep out of reach of children: If accidentally ingested, get medical help or contact a Poison Control Center immediately.
Do not use
● Do not use with other ointments, creams, sprays or liniments
●Do not use with heating pad or device.
When using this product:
● Avoid contact with the eyes or mucous membranes
● Do not apply to wounds or damaged skin
● Do not use with other ointments, creams, sprays or liniments
● Do not apply to irritated skin or if excessive irritation develops
● Do not bandage
● Wash hands after use with cool water
● Do not use with heating pad or device.
Stop use and ask a doctor if: Condition worsens, or if symptoms persist for more than 7 days, or clear up and reoccur.
If pregnant or breast-feeding: Ask a health professional before use.
Keep out of reach of children: If accidentally ingested, get medical help or contact a Poison Control Center immediately.
Directions
Adults and children 12 years of age and older:
● apply generously to affected area
● massage into painful area until thoroughly absorbed into skin
● repeat as necessary ,but no more than 4 times daily .
Children 12 years or younger: Consult physician.
Other information
Store at room temperature with the cap closed
Notice: because this product contains natural nutrients,color may vary.