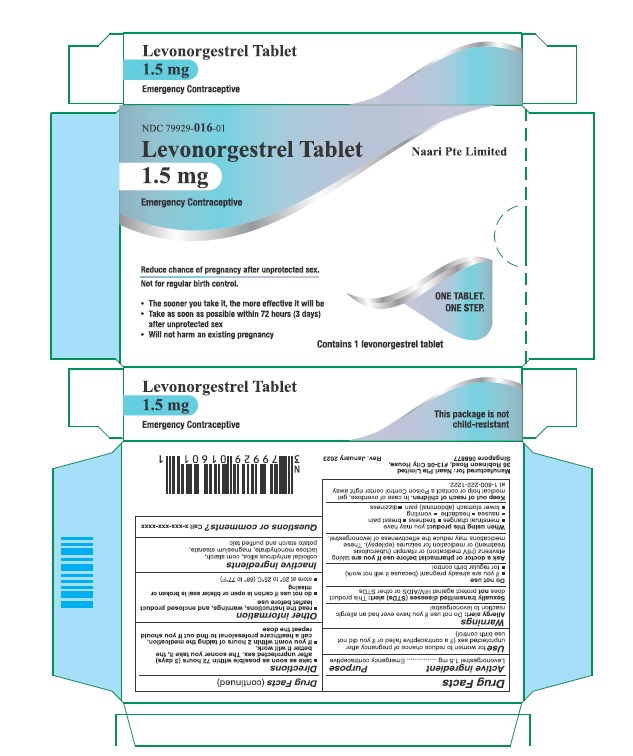
Use
for women to reduce chance of pregnancy after unprotected sex (if a contraceptive failed or if you did not use birth control)
Warnings
Sexually transmitted diseases (STDs) alert
This product does not protect against HIV/AIDS or other STDs
Ask a doctor or pharmacist before use if you are taking efavirenz (HIV medication) or rifampin (tuberculosis treatment) or medication for seizures (epilepsy). These medications may reduce the effectiveness of levonorgestrel.
Directions
- take as soon as possible within 72 hours (3 days) after unprotected sex. The sooner you take it the better it will work.
- if you vomit within 2 hours after taking the medication, call a healthcare professional to find out if you should repeat the dose
Other information
- read the instructions, warnings and enclosed product leaflet before use
- do not use if carton is open or tear strip is removed or blister seal is broken or missing
- store at 20-25°C (68-77°F)
Inactive ingredients
colloidal anhydrous silica, corn starch, lactose monohydrate, magnesium stearate, potato starch and purified talc
Questions?
If you have questions or need more information, call our toll-free number, x-xxx-xxx-xxxx.