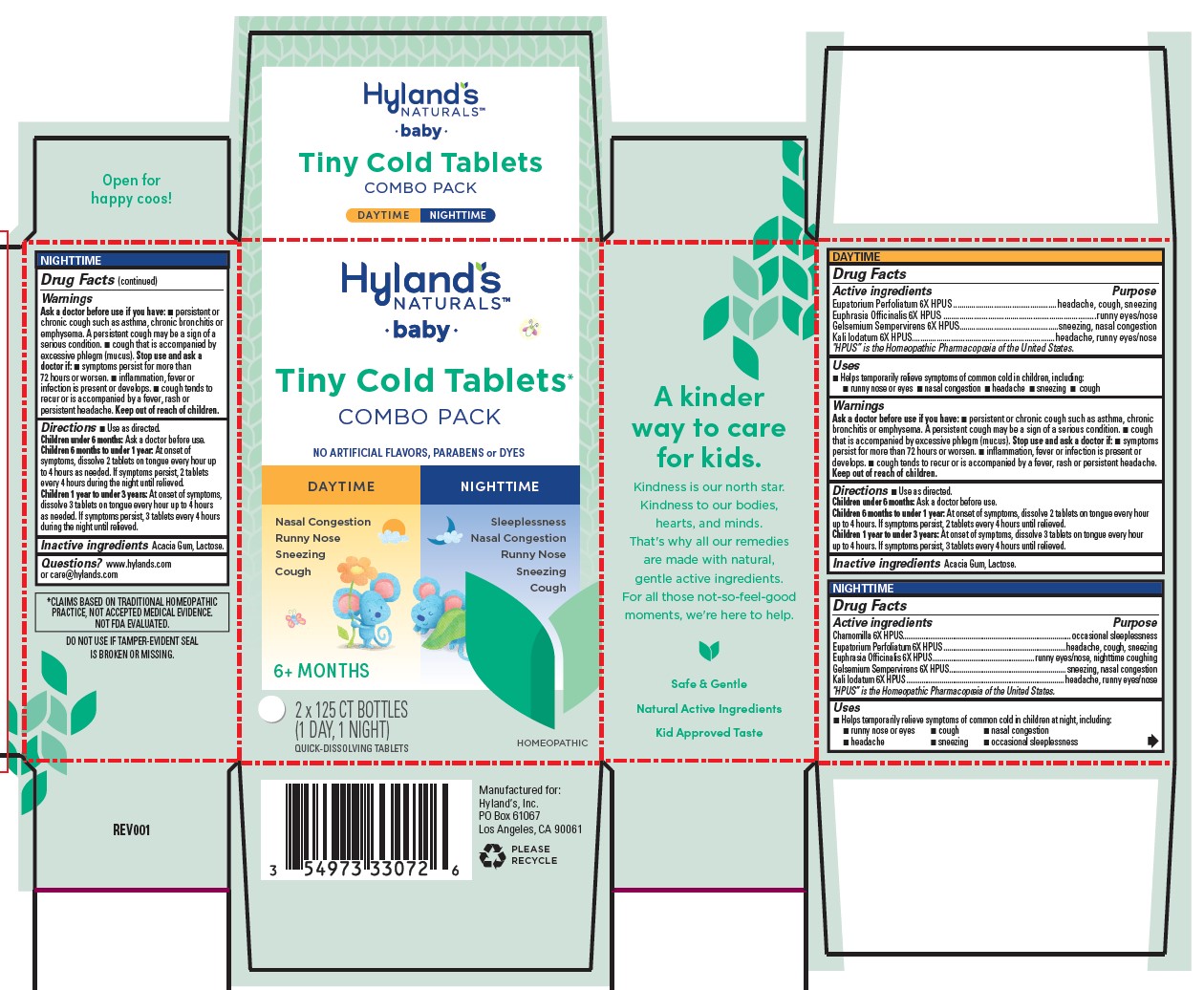
| Active ingredients | Purpose |
| Eupatorium Perfoliatum 6X HPUS | headache, cough, sneezing |
| Euphrasia Officinalis 6X HPUS | runny eyes/nose |
| Gelsemium Sempervirens 6X HPUS | sneezing, nasal congestion |
| Kali Iodatum 6X HPUS | headache, runny eyes/nose |
"HPUS” is the Homeopathic Pharmacopoeia of the United States.
Uses
■ Helps temporarily relieve symptoms of common cold in children, including: ■ runny nose or eyes ■ nasal congestion ■ headache ■ sneezing ■ cough
Ask a doctor before use if you have
■ persistent or chronic cough such as asthma, chronic bronchitis or emphysema. A persistent cough may be a sign of a serious condition. ■ cough that is accompanied by excessive phlegm (mucus).
Stop use and ask a doctor if
■ symptoms persist for more than 72 hours or worsen. ■ inflammation, fever or infection is present or develops. ■ cough tends to recur or is accompanied by a fever, rash or persistent headache.
Directions
■ Use as directed.
| Children under 6 months: | Ask a doctor before use. |
| Children 6 months to under 1 year: | At onset of symptoms, dissolve 2 tablets on tongue every hour
up to 4 hours. If symptoms persist, 2 tablets every 4 hours until relieved. |
| Children 1 year to under 3 years: | At onset of symptoms, dissolve 3 tablets on tongue every hour
up to 4 hours. If symptoms persist, 3 tablets every 4 hours until relieved. |
| Active ingredients | Purpose |
| Chamomilla 6X HPUS | occasional sleeplessness |
| Eupatorium Perfoliatum 6X HPUS | headache, cough, sneezing |
| Euphrasia Officinalis 6X HPUS | runny eyes/nose, nighttime coughing |
| Gelsemium Sempervirens 6X HPUS | sneezing, nasal congestion |
| Kali Iodatum 6X HPUS | headache, runny eyes/nose |
"HPUS” is the Homeopathic Pharmacopoeia of the United States.
Uses
■ Helps temporarily relieve symptoms of common cold in children at night, including:
■ runny nose or eyes ■ cough ■ nasal congestion
■ headache ■ sneezing ■ occasional sleeplessness
Ask a doctor before use if you have
■ persistent or chronic cough such as asthma, chronic bronchitis or emphysema. A persistent cough may be a sign of a serious condition. ■ cough that is accompanied by excessive phlegm (mucus).
Stop use and ask a doctor if
■ symptoms persist for more than 72 hours or worsen. ■ inflammation, fever or infection is present or develops. ■ cough tends to
recur or is accompanied by a fever, rash or persistent headache.
Directions
■ Use as directed
| Children under 6 months: | Ask a doctor before use. |
| Children 6 months to under 1 year: | At onset of symptoms, dissolve 2 tablets on tongue every hour up to 4 hours as needed. If symptoms persist, 2 tablets every 4 hours during the night until relieved. |
| Children 1 year to under 3 years: | At onset of symptoms, dissolve 3 tablets on tongue every hour up to 4 hours as needed. If symptoms persist, 3 tablets every 4 hours during the night until relieved. |
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
Helps temporarily relieve symptoms of common cold in children at night, including: runny nose or eyes, cough, nasal congestion,
headache, sneezing, occasional sleeplessness.
Principal Display Panel
Hyland's
NATURALS
•baby•
Tiny Cold Tablets*
COMBO PACK
NO ARTIFICIAL FLAVORS, PARABENS or DYES
DAYTIME
Nasal Congestion
Runny Nose
Sneezing
Cough
NIGHTTIME
Sleeplessness
Nasal Congestion
Runny Nose
Sneezing
Cough
6+ MONTHS
2 x 125 CT BOTTLES
(1 DAY, 1 NIGHT)
QUICK-DISSOLVING TABLETS
HOMEOPATHIC