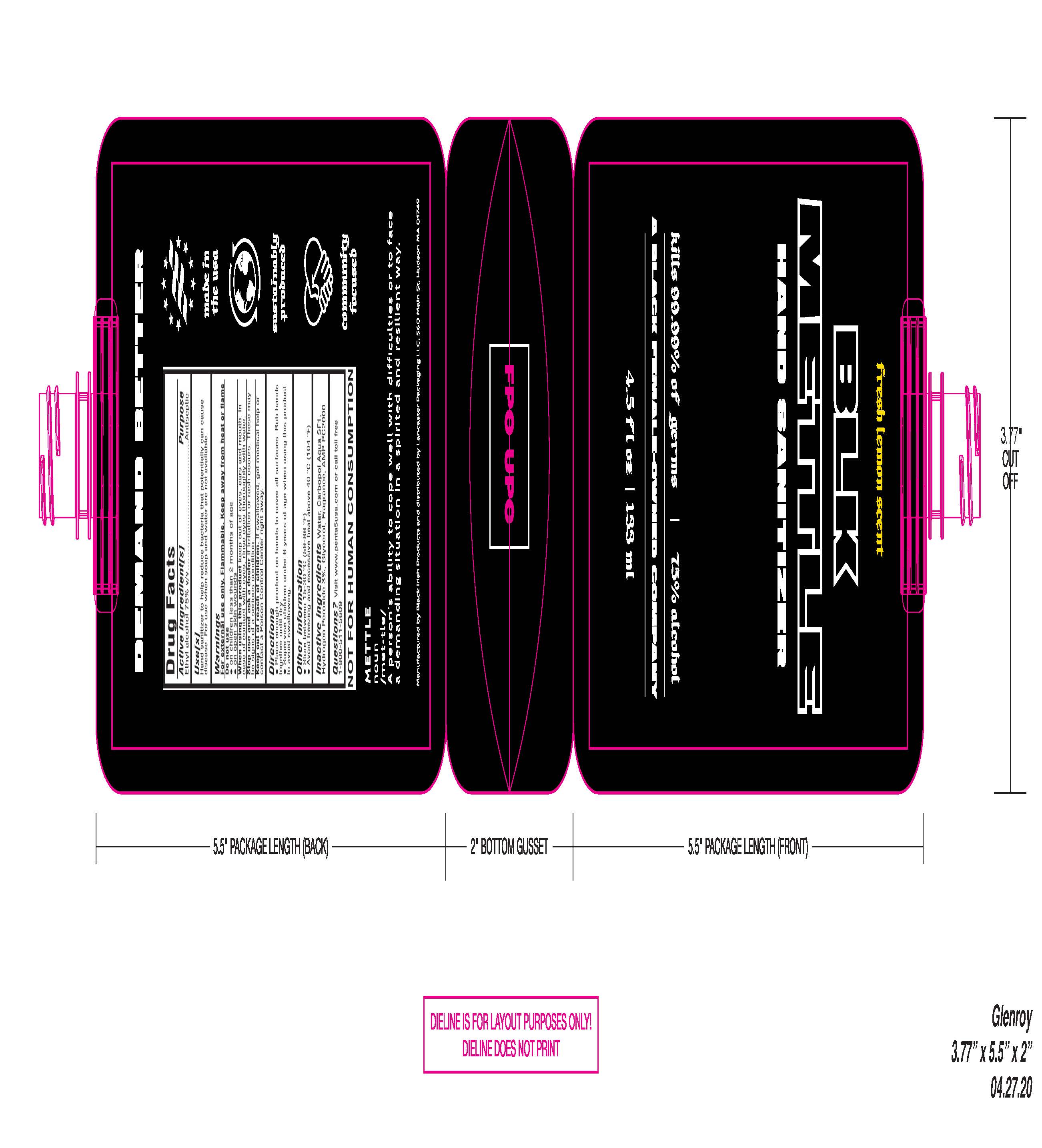
HAND SANITIZER- ethyl alcohol gel
Black Irish Products, LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Ethyl Alcohol 75% v/v Purpose: Antiseptic
Keep out of eyes, ears, and mouth. In case of contact with the eyes, rinse eyes thoroughly with water.
- Place enough product on hands to cover all surfaces. Rub hands together until dry.
- Supervise with children under 6 years of age when using this product to avoid swallowing.
- on chilren less then 2 months of age
- on open skin wounds
if irritation or rash occurs. These may be signs of a serious condition.
If swallowed, get medical help or contact a Poison Control Center right away
Apply dime size amount to hands and
Hand sanitizer to help reuce bacteria that potentially can cause disease. For use when soap and water are not available.
Visit penta5usa.com or call toll free 1-800-511-5609
- Store between 15-30 Degrees C (59-86 F)
- Avoid freexing and excessive heat about 40 C (104 F)
Water, Carbopol Aqua SF1, Hydrogen Peroxide 3%, Glycerol, Fragrance, AMP PC2000
 236 ML, NDC: 80589-001-01
236 ML, NDC: 80589-001-01
59 mL NDC 80589-001-02

3785 mL, NDC 80589-001-03

60 mL NDC 80589-001-04

133 mL NDC 80589-001-05
Black Irish Products, LLC.