FULL PRESCRIBING INFORMATION
1 INDICATIONS & USAGE
Travoprost Ophthalmic Solution USP, 0.004% is indicated for the reduction of elevated intraocular pressure in patients with open angle glaucoma or ocular hypertension.
2 DOSAGE & ADMINISTRATION
The recommended dosage is one drop in the affected eye(s) once daily in the evening. Travoprost Ophthalmic Solution USP, 0.004% should not be administered more than once daily since it has been shown that more frequent administration of prostaglandin analogs may decrease the intraocular pressure lowering effect.
Reduction of the intraocular pressure starts approximately 2 hours after the first administration with maximum effect reached after 12 hours.
Travoprost ophthalmic solution may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart.
5 WARNINGS AND PRECAUTIONS
5.1 Pigmentation
Travoprost ophthalmic solution has been reported to cause changes to pigmented tissues. The most frequently reported changes have been increased pigmentation of the iris, periorbital tissue (eyelid) and eyelashes. Pigmentation is expected to increase as long as travoprost is administered. The pigmentation change is due to increased melanin content in the melanocytes rather than to an increase in the number of melanocytes. After discontinuation of travoprost, pigmentation of the iris is likely to be permanent, while pigmentation of the periorbital tissue and eyelash changes have been reported to be reversible in some patients. Patients who receive treatment should be informed of the possibility of increased pigmentation. The long term effects of increased pigmentation are not known.
Iris color change may not be noticeable for several months to years. Typically, the brown pigmentation around the pupil spreads concentrically towards the periphery of the iris and the entire iris or parts of the iris become more brownish. Neither nevi nor freckles of the iris appear to be affected by treatment. While treatment with travoprost ophthalmic solution can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly. (see PATIENT COUNSELING INFORMATION, 17.1).
5.2 Eyelash Changes
Travoprost ophthalmic solution may gradually change eyelashes and vellus hair in the treated eye. These changes include increased length, thickness, and number of lashes. Eyelash changes are usually reversible upon discontinuation of treatment.
5.3 Intraocular Inflammation
Travoprost ophthalmic solution should be used with caution in patients with active intraocular inflammation (e.g., uveitis) because the inflammation may be exacerbated.
5.4 Macular Edema
Macular edema, including cystoid macular edema, has been reported during treatment with travoprost ophthalmic solution. Travoprost ophthalmic solution should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
5.5 Angle-closure, Inflammatory or Neovascular Glaucoma
Travoprost ophthalmic solution has not been evaluated for the treatment of angle-closure, inflammatory or neovascular glaucoma.
5.6 Bacterial Keratitis
There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface (see PATIENT COUNSELING INFORMATION, 17.3).
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The most common adverse reaction observed in controlled clinical studies with travoprost ophthalmic solution 0.004% was ocular hyperemia which was reported in 30 to 50% of patients. Up to 3% of patients discontinued therapy due to conjunctival hyperemia. Ocular adverse reactions reported at an incidence of 5 to 10% in these clinical studies included decreased visual acuity, eye discomfort, foreign body sensation, pain and pruritus.
Ocular adverse reactions reported at an incidence of 1 to 4% in clinical studies with travoprost ophthalmic solution 0.004% included abnormal vision, blepharitis, blurred vision, cataract, conjunctivitis, corneal staining, dry eye, iris discoloration, keratitis, lid margin crusting, ocular inflammation, photophobia, subconjunctival hemorrhage and tearing.
Nonocular adverse reactions reported at an incidence of 1 to 5% in these clinical studies were allergy, angina pectoris, anxiety, arthritis, back pain, bradycardia, bronchitis, chest pain, cold/flu syndrome, depression, dyspepsia, gastrointestinal disorder, headache, hypercholesterolemia, hypertension, hypotension, infection, pain, prostate disorder, sinusitis, urinary incontinence and urinary tract infections.
In post-marketing use with prostaglandin analogs, periorbital and lid changes including deepening of the eyelid sulcus have been observed.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Teratogenic effects: Travoprost was teratogenic in rats, at an intravenous (IV) dose up to 10 mcg/kg/day (250 times the maximal recommended human ocular dose (MRHOD), evidenced by an increase in the incidence of skeletal malformations as well as external and visceral malformations, such as fused sternebrae, domed head and hydrocephaly. Travoprost was not teratogenic in rats at IV doses up to 3 mcg/kg/day (75 times the MRHOD), or in mice at subcutaneous doses up to 1 mcg/kg/day (25 times the MRHOD). Travoprost produced an increase in post-implantation losses and a decrease in fetal viability in rats at IV doses >3 mcg/kg/day (75 times the MRHOD) and in mice at subcutaneous doses >0.3 mcg/kg/day (7.5 times the MRHOD).
In the offspring of female rats that received travoprost subcutaneously from Day 7 of pregnancy to lactation Day 21 at doses of ≥0.12 mcg/kg/day (3 times the MRHOD), the incidence of postnatal mortality was increased, and neonatal body weight gain was decreased. Neonatal development was also affected, evidenced by delayed eye opening, pinna detachment and preputial separation, and by decreased motor activity.
There are no adequate and well-controlled studies of travoprost ophthalmic solution 0.004% administration in pregnant women. Because animal reproductive studies are not always predictive of human response, travoprost ophthalmic solution should be administered during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
A study in lactating rats demonstrated that radiolabeled travoprost and/or its metabolites were excreted in milk. It is not known whether this drug or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when travoprost ophthalmic solution is administered to a nursing woman.
8.4 Pediatric Use
Use in pediatric patients below the age of 16 years is not recommended because of potential safety concerns related to increased pigmentation following long-term chronic use.
11 DESCRIPTION
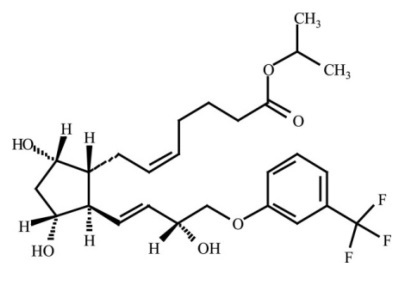
Travoprost is a synthetic prostaglandin F analogue. Its chemical name is [1R-[1α(Z),2β(1E,3R*),3α,5α]]-7-[3,5Dihydroxy-2-[3-hydroxy-4-[3(trifluoromethyl)phenoxy]-1-butenyl]cyclopentyl]-5heptenoic acid, 1-methylethylester. It has a molecular formula of C26H35F3O6 and a molecular weight of 500.55. The chemical structure of travoprost is:
Travoprost is a clear, colorless and viscous oil that is very soluble in acetonitrile, methanol, octanol, and chloroform. It is practically insoluble in water.
Travoprost Ophthalmic Solution 0.004% is supplied as sterile, buffered aqueous solution of travoprost with a pH of approximately 6.0 and an osmolality of approximately 290 mOsmol/kg.
Travoprost ophthalmic solution contains Active: travoprost 0.04 mg/mL; Preservative: benzalkonium chloride 0.015% w/v; Inactives: polyoxyl 40 hydrogenated castor oil, tromethamine, boric acid, mannitol, edetate disodium, sodium hydroxide and/or hydrochloric acid (to adjust pH)and water for injection.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Travoprost free acid, a prostaglandin analog is a selective FP prostanoid receptor agonist which is believed to reduce intraocular pressure by increasing uveoscleral outflow. The exact mechanism of action is unknown at this time.
12.3 Pharmacokinetics
Travoprost is absorbed through the cornea and is hydrolyzed to the active free acid. Data from four multiple dose pharmacokinetic studies (totaling 107 subjects) have shown that plasma concentrations of the free acid are below 0.01 ng/mL (the quantitation limit of the assay) in two-thirds of the subjects. In those individuals with quantifiable plasma concentrations (N=38), the mean plasma Cmax was 0.018 ± 0.007 ng/mL (ranged 0.01 to 0.052 ng/mL) and was reached within 30 minutes. From these studies, travoprost is estimated to have a plasma half-life of 45 minutes. There was no difference in plasma concentrations between Days 1 and 7, indicating steady-state was reached early and that there was no significant accumulation.
Travoprost, an isopropyl ester prodrug, is hydrolyzed by esterases in the cornea to its biologically active free acid. Systemically, travoprost free acid is metabolized to inactive metabolites via betaoxidation of the α(carboxylic acid) chain to give the 1,2-dinor and 1,2,3,4-tetranor analogs, via oxidation of the 15-hydroxyl moiety, as well as via reduction of the 13,14 double bond.
The elimination of travoprost free acid from plasma was rapid and levels were generally below the limit of quantification within one hour after dosing. The terminal elimination half-life of travoprost free acid was estimated from fourteen subjects and ranged from 17 minutes to 86 minutes with the mean halflife of 45 minutes. Less than 2% of the topical ocular dose of travoprost was excreted in the urine within 4 hours as the travoprost free acid.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
Two-year carcinogenicity studies in mice and rats at subcutaneous doses of 10, 30, or 100 mcg/kg/day did not show any evidence of carcinogenic potential. However, at 100 mcg/kg/day, male rats were only treated for 82 weeks, and the maximum tolerated dose (MTD) was not reached in the mouse study. The high dose (100 mcg/kg) corresponds to exposure levels over 400 times the human exposure at the maximum recommended human ocular dose (MRHOD) of 0.04 mcg/kg, based on plasma active drug levels.
Travoprost was not mutagenic in the Ames test, mouse micronucleus test or rat chromosome aberration assay. A slight increase in the mutant frequency was observed in one of two mouse lymphoma assays in the presence of rat S-9 activation enzymes.
Travoprost did not affect mating or fertility indices in male or female rats at subcutaneous doses up to 10 mcg/kg/day [250 times the maximum recommended human ocular dose of 0.04 mcg/kg/day on a mcg/kg basis (MRHOD)]. At 10 mcg/kg/day, the mean number of corpora lutea was reduced, and the post-implantation losses were increased. These effects were not observed at 3 mcg/kg/day (75 times the MRHOD).
14 CLINICAL STUDIES
In clinical studies, patients with open-angle glaucoma or ocular hypertension and baseline pressure of 25 to 27 mm Hg who were treated with travoprost ophthalmic solution 0.004% dosed once-daily in the evening demonstrated 7 to 8 mm Hg reductions in intraocular pressure. In subgroup analyses of these studies, mean IOP reduction in black patients was up to 1.8 mm Hg greater than in non-black patients. It is not known at this time whether this difference is attributed to race or to heavily pigmented irides.
In a multi-center, randomized, controlled trial, patients with mean baseline intraocular pressure of 24 to 26 mm Hg on TIMOPTIC* 0.5% BID who were treated with travoprost ophthalmic solution 0.004% dosed QD adjunctively to TIMOPTIC* 0.5% BID demonstrated 6 to 7 mm Hg reductions in intraocular pressure.
16 HOW SUPPLIED/STORAGE AND HANDLING
Travoprost Ophthalmic Solution 0.004% is a sterile, isotonic, buffered, preserved, aqueous solution of travoprost (0.04 mg/mL).
Travoprost ophthalmic solution is supplied as a 2.5 mL and a 5 mL solution in a 5 mL screw neck bottle made with natural polypropylene, nozzle made with low density polyethylene and turquoise color screw cap made with high density polyethylene. Tamper evidence is provided with a tamper evident ring around the closure and neck area of the package.
2.5 mL fill NDC 62332-510-25
5 mL fill NDC 62332-510-05
Storage
Store at 2º to 25ºC (36º to 77ºF).
17 PATIENT COUNSELING INFORMATION
17.1 Potential for Pigmentation
Patients should be advised about the potential for increased brown pigmentation of the iris, which may be permanent. Patients should also be informed about the possibility of eyelid skin darkening, which may be reversible after discontinuation of travoprost ophthalmic solution 0.004%.
17.2 Potential for Eyelash Changes
Patients should also be informed of the possibility of eyelash and vellus hair changes in the treated eye during treatment with travoprost ophthalmic solution. These changes may result in a disparity between eyes in length, thickness, pigmentation, number of eyelashes or vellus hairs, and/or direction of eyelash growth. Eyelash changes are usually reversible upon discontinuation of treatment.
17.3 Handling the Container
Patients should be instructed to avoid allowing the tip of the dispensing container to contact the eye, surrounding structures, fingers, or any other surface in order to avoid contamination of the solution by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
17.4 When to Seek Physician Advice
Patients should also be advised that if they develop an intercurrent ocular condition (e.g., trauma or infection), have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician’s advice concerning the continued use of travoprost ophthalmic solution.
17.5 Use with Contact Lenses
Patients should be advised that travoprost ophthalmic solution contains benzalkonium chloride, which may be absorbed by soft contact lenses. Contact lenses should be removed prior to instillation of travoprost ophthalmic solution and may be reinserted 15 minutes following its administration.
17.6 Use with Other Ophthalmic Drugs
If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes between applications.
* TIMOPTIC is the registered trademark of Merck & Co., Inc.
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA
Made in India.
Manufactured by:
Gland Pharma Limited
Hyderabad - 500 043, INDIA.
Revised: 07/2022
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
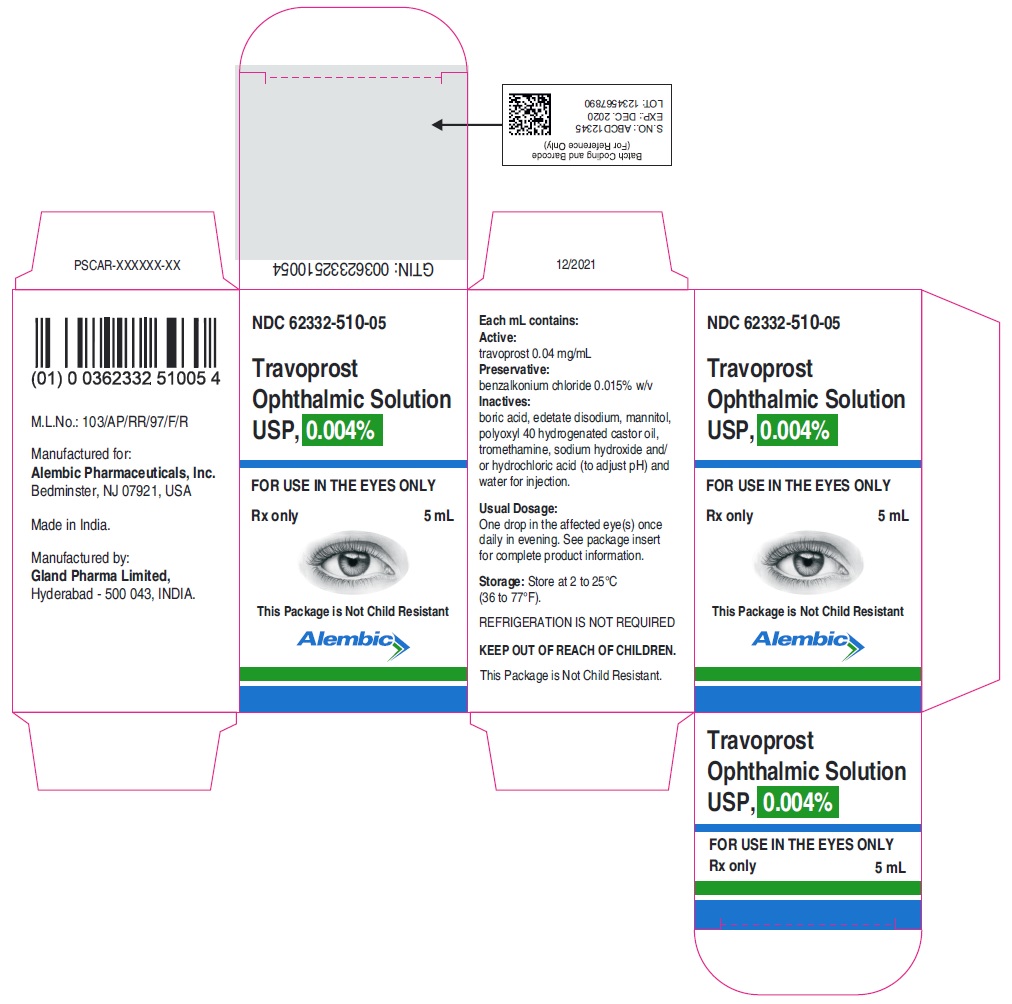
PRINCIPAL DISPLAY PANEL – Travoprost Ophthalmic Solution USP 0.004%, 2.5 mL Fill Container

PRINCIPAL DISPLAY PANEL – Travoprost Ophthalmic Solution USP 0.004%, 2.5 mL Fill Carton

PRINCIPAL DISPLAY PANEL – Travoprost Ophthalmic Solution USP 0.004%, 5.0 mL Fill Container

PRINCIPAL DISPLAY PANEL – Travoprost Ophthalmic Solution USP 0.004%, 5.0 mL Fill Carton