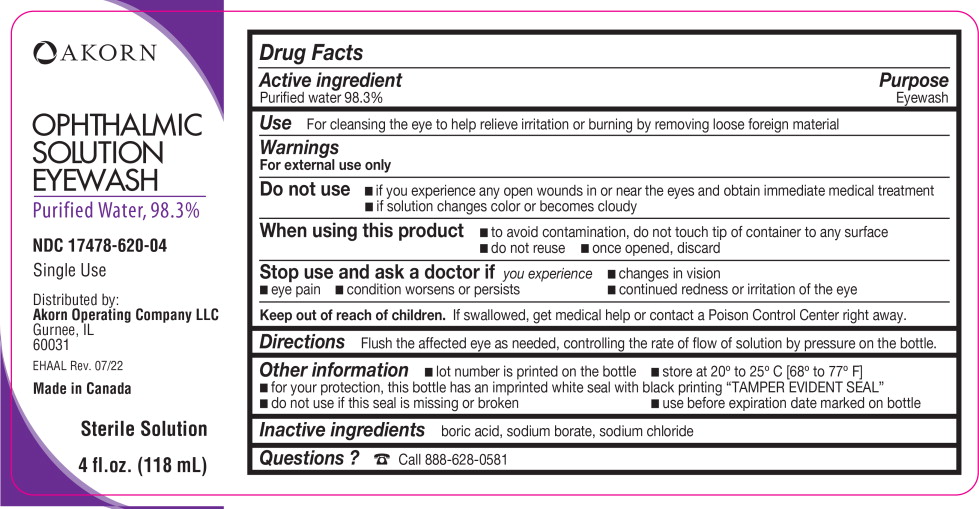
Active ingredient
Purified water 98.3%
Use
For cleansing the eye to help relieve irritation or burning by removing loose foreign material
Warnings
For external use only
Do not use
- if you experience any open wounds in or near the eyes and obtain immediate medical treatment
- if solution changes color or becomes cloudy
When using this product
- to avoid contamination, do not touch tip of container to any surface
- do not reuse
- once opened, discard
Stop use and ask a doctor if
you experience
- changes in vision
- eye pain
- condition worsens or persists
- continued redness or irritation of the eye
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Flush the affected eye as needed, controlling the rate of flow of solution by pressure on the bottle.
Other information
- lot number is printed on the bottle
- store at 20° to 25° C [68° to 77° F]
- for your protection, this bottle has an imprinted white seal with black printing “TAMPER EVIDENT SEAL”
- do not use if this seal is missing or broken
- use before expiration date marked on bottle
Inactive ingredients
boric acid, sodium borate, sodium chloride
Questions?
Call 888-628-0581
Principal Display Panel Text for Container Label:
Akorn logo
OPHTHALMIC
SOLUTION
EYEWASH
Purified Water, 98.3%
NDC 17478-620-04
Single Use
Distributed by:
Akorn Operating Company LLC
Gurnee, IL
60031
EHAAL Rev. 07/22
Made in Canada
Sterile Solution
4 fl.oz. (118 mL)